Chapter 2 Properties of Matter Classifying Matter Physical













- Slides: 22

Chapter 2 Properties of Matter Classifying Matter Physical Properties Chemical Properties

What is Matter? • Anything that takes up space and has mass.

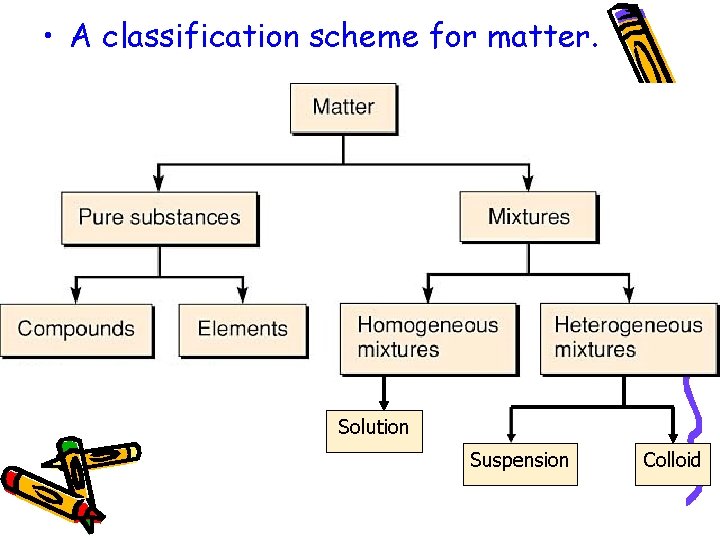
There are different kinds of Matter • Pure Substances – Elements – Compounds • Mixtures – Solutions – Colloids – Suspensions

• A classification scheme for matter. Solution Suspension Colloid

Pure Substances • A substance is either an element or a compound. • Always has exactly the same composition. • Every sample has the same properties because it has a fixed, uniform composition.

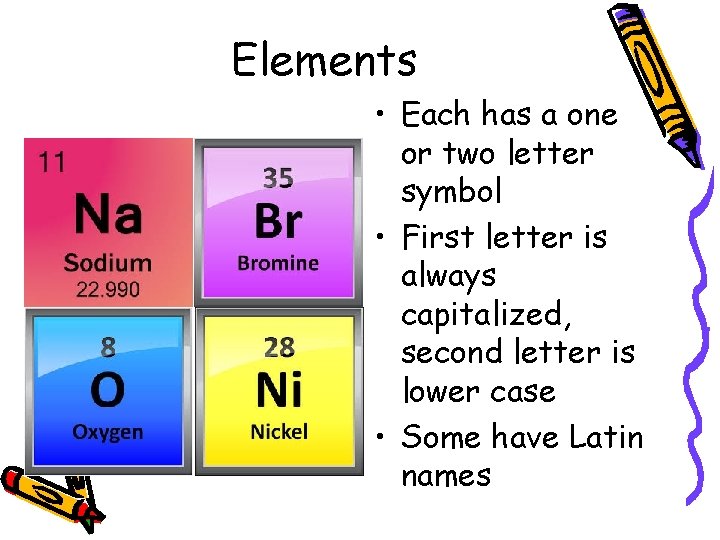
Elements • If all the atoms in a substance are alike—then you are observing an element. • 90 elements are naturally occurring, most are unstable.

Elements • Each has a one or two letter symbol • First letter is always capitalized, second letter is lower case • Some have Latin names

Compounds • Materials that are made up of 2 or more elements combined in a definite or fixed proportion. (i. e. , salt, sugar, water) • The ratio of different atoms, is always the same. Water (H 2 O) = 2 -H + 1 -0. • Compounds, generally, take on characteristics of their own and do not resemble the individual elements that compose them. • Can elements and compounds be separated by physical means? NO

Mixtures • A mixture is two or more substances mixed together, that can be separated by physical means (i. e. , salt, water). • Composition varies. • Two main types of mixtures: – Heterogeneous – Homogeneous

Homogeneous Mixtures • Two or more substances blended evenly throughout. • Also called solutions, which remain constantly and uniformly mixed. • Examples: – Soda – Vinegar

Heterogeneous Mixtures • Do not always contain the same proportions of each ingredient. • Not every part of the mixture has the same composition. • Examples: – Granite – Dry soup mix – Trail mix

Suspension • Heterogeneous mixtures. • Generally, there is a liquid with some sort of “stuff” that can settle out.

Colloids • Colloids never settle. • They are Heterogeneous Mixtures. • These particles are big enough to scatter light. • Examples: – Milk – Fog – paint

Fog • A colloid composed of water droplets suspended in the air. • Particles in the air can scatter the light, making fog. (hard to see through) • The scattering is known as the “Tyndall Effect”.

Physical Properties • A physical property of a material is a characteristic that can be observed without changing the substances that make up or compose the material.

Physical Changes • Change in: – Shape – State of matter (i. e. , breaking chalk, freezing) • When a physical change occurs, the actual substance does not change.

Some Physical Properties • Describes: – – – – – Appearance (Color, Shape) Measurements (Size) Density Melting point (State of Matter) Boiling point (State of Matter) Viscosity Conductivity Malleability Hardness

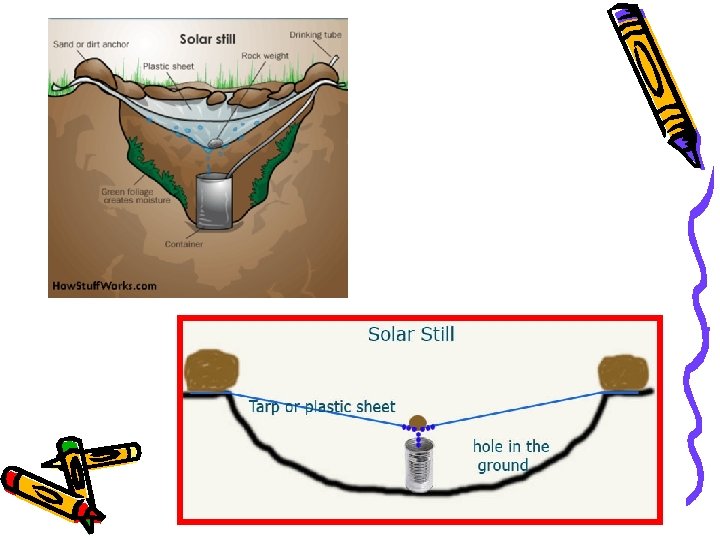
Using Physical Properties • Physical properties can be used to separate mixtures. - Filtration - Distillation


Chemical Change • The changing of one substance to another substance. • Change in: – – Smell Foaming Light, sound, or heat Examples—rusting & burning - Flammability Reactivity Change in color (Sometimes) Formation of a precipitate

Chemical Properties • Are only seen when the substance is changing into a different substance.

Conservation of Mass • Matter cannot be created or destroyed. • A burning log. Where does the mass go?