Classifying Matter Understanding Matter Matter is anything that





































- Slides: 45

Classifying Matter

Understanding Matter • Matter is anything that has mass and takes up space. • Obviously, there are different types of matter. • Metal, wood, plastic, your flesh are all obvious pieces of matter.

Understanding Matter • Air is also matter. • Air molecules have mass and take up space. • Sound and light are not matter. • Forces and energy are not matter. • To be matter, something must have mass and take up space.

Atoms • An atom is a small particle that is a building block of matter.

Atoms • An atom is almost too small to even imagine. • Look at a piece of human hair. • The diameter, or distance across the hair on its smallest side is still 1, 000 times bigger than an atom.

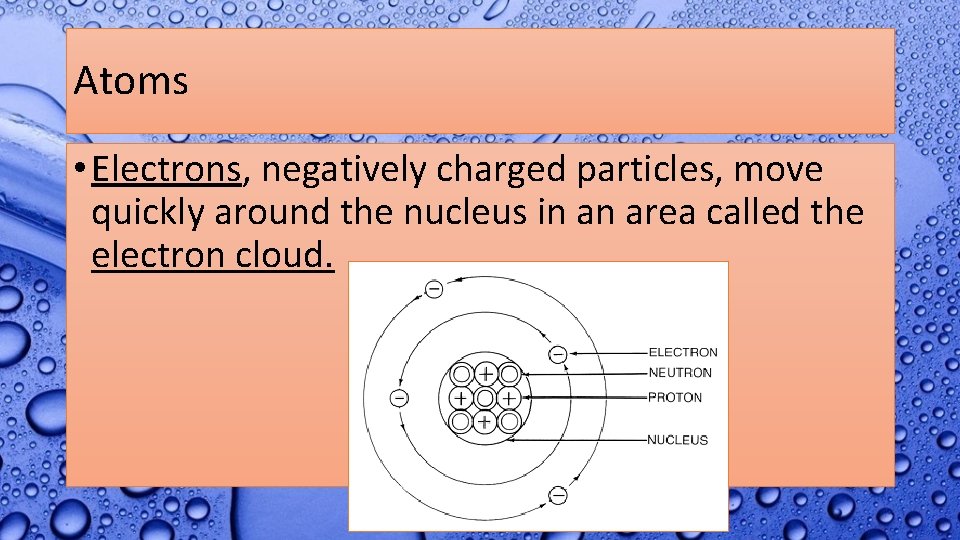
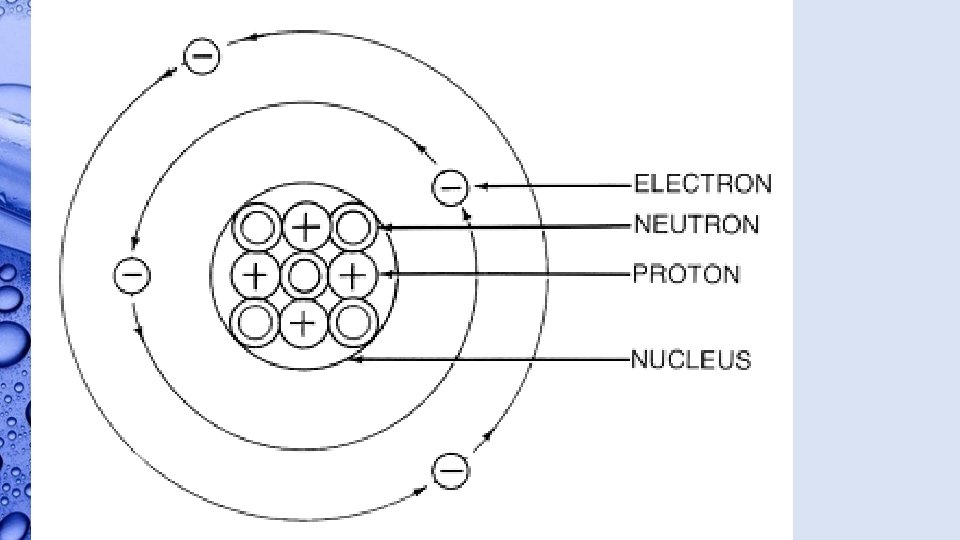
Atoms • Atoms are made of three parts. • The center of the atom is called the nucleus. • Protons, positively charged particles, and neutrons, neutrally charged particles, make up the nucleus.

Atoms • Electrons, negatively charged particles, move quickly around the nucleus in an area called the electron cloud.


Atoms • Not all atoms have the same number of protons, neutrons, and electrons. • Having different numbers of protons cause these atoms to have different properties and act in different ways.

Substances • A substance is matter with a composition that is always the same. • This means that a given substance is always made up of the same combinations of atoms.

Substances • Any piece of aluminum is made of the same type of atoms. • Water is always made of hydrogen and oxygen atoms in a certain combination. • Anything else would not be water.

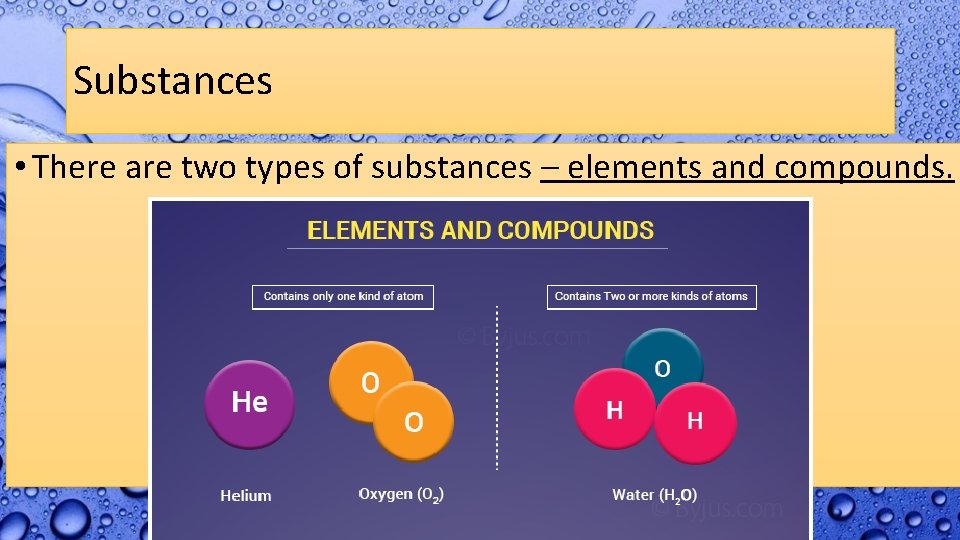
Substances • There are two types of substances – elements and compounds.

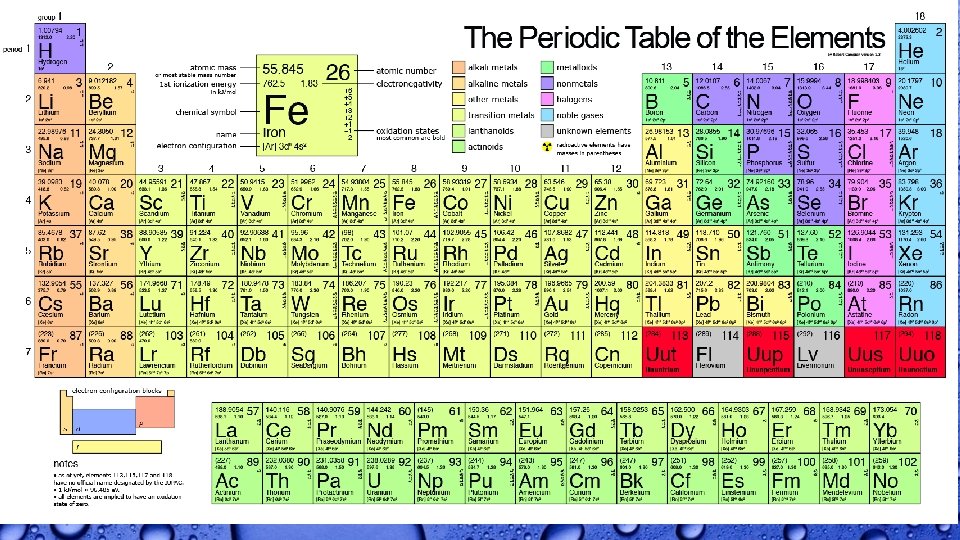
Elements • An element is a substance that consists of just one type of atom. • The periodic table of elements is a way we’ve organized the types of atoms we have discovered. • There are 118 different elements known because there are 118 different types of atoms known.


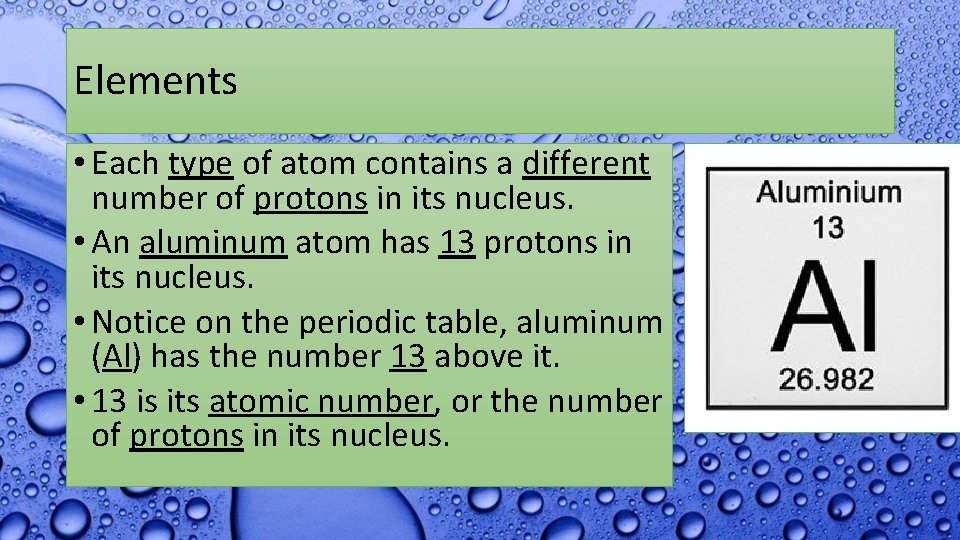
Elements • Each type of atom contains a different number of protons in its nucleus. • An aluminum atom has 13 protons in its nucleus. • Notice on the periodic table, aluminum (Al) has the number 13 above it. • 13 is its atomic number, or the number of protons in its nucleus.

Elements • The symbol for aluminum is Al. • A chemical symbol is either one capitalized letter or one capitalized letter with a lower case letter after it.

Elements • Most elements can exist as individual atoms. • For example, a roll of pure aluminum foil is made of trillions of individual aluminum atoms.

Elements • Some atoms, however, exist in groups. • Oxygen atoms in the air exist as pairs. • Oxygen atoms will not exist by themselves if even one other oxygen atom is nearby.

Elements • Whether the atoms of an element exist individually like aluminum or in groups like oxygen, an element contains only one type of atom. • It’s composition is always the same.

Compounds • A compound is a type of substance that contains atoms of two or more different elements chemically bonded together. (We’ll talk about chemical bonds later)

Compounds • Carbon dioxide (CO₂) is a compound. • It is made of carbon atoms and oxygen atoms. • These are different elements bonded together chemically. • Carbon dioxide is a substance because the C and the O atoms are always combined in the same way.

Compounds – Chemical Formulas • The combination of symbols and numbers that represent a compound is called a chemical formula. • Chemical formulas show the different atoms that make up a compound, using their element symbols.

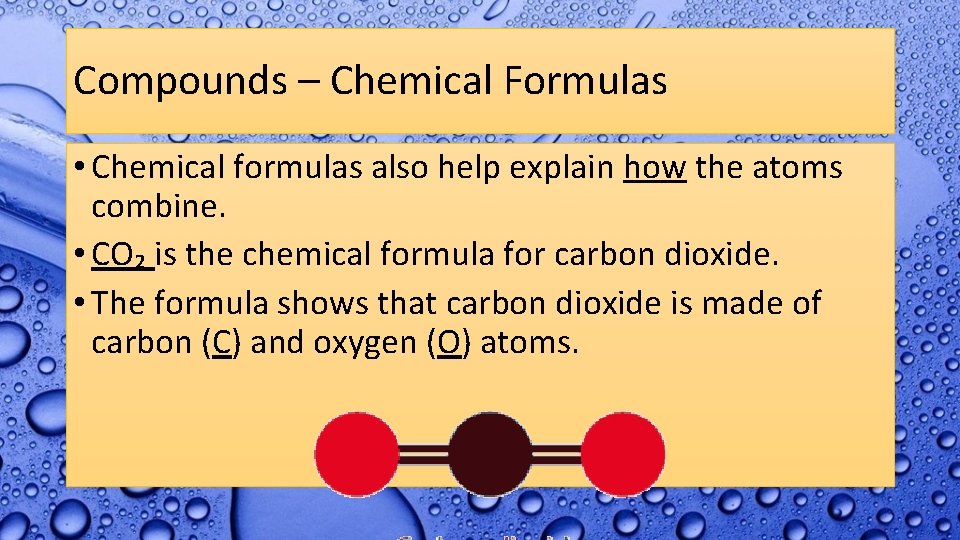
Compounds – Chemical Formulas • Chemical formulas also help explain how the atoms combine. • CO₂ is the chemical formula for carbon dioxide. • The formula shows that carbon dioxide is made of carbon (C) and oxygen (O) atoms.

Compounds – Chemical Formulas • The small ₂ is called a subscript. • This means that in CO₂, there are two oxygen atoms. • The ₂ is attached to the O. • There is only one carbon atom because there is no subscript written after the symbol.

Compounds - Chemical Formulas • Practice: Identify what types of atoms and how many of each are in each compound. 1) H₂O (water)

Compounds - Chemical Formulas 2) HCl (Hydrochloric acid)

Compounds - Chemical Formulas 3) C 6 H 12 O 6 (Glucose sugar)

Compounds – Properties of Compounds • A compound often has different properties than the individual elements it is made of.

Compounds – Properties of Compounds • Na. Cl is a common compound. • Sodium, Na, is a soft silvery metal that will explode in the presence of water.

Compounds – Properties of Compounds • Chlorine, or Cl, is a highly toxic yellow-green gas.

Compounds – Properties of Compounds • When Na and Cl form a chemical bond, they make Na. Cl. • Na. Cl is table salt. • Obviously the properties of the original elements have changed during the chemical bond.

Elements and Compounds • Both elements and compounds are considered substances. • Remember, a substance is matter with a composition that is always the same.

Elements and Compounds • An element is a substance because it is only made of one type of atom. • A compound is a substance because that compound MUST have those elements in those amounts or proportions. • All substances, elements or compounds, have their own unique properties.

Mixtures • A mixture is NOT a substance. • A mixture is matter that can vary in composition. • Mixtures are combinations of two or more substances that have been physically blended (or mixed) together.

Mixtures • A mixture is not a substance, so the amounts of the substances do not have to be the same. • The amounts can also differ throughout the mixture.

Mixtures • Think about water mixing with sand. • The water and the sand do not chemically bond. • They form a mixture. • Because they aren’t chemically combined as compounds, they can be separated again through physical methods, like filtering.

Mixtures – Heterogeneous • A heterogeneous mixture is a type of mixture in which the individual substances are not evenly mixed. • Because they aren’t evenly mixed, two samples from the same substance can appear completely different from each other.

Mixtures – Heterogeneous • Trail mix is a heterogeneous mixture. • Different substances are mixed together, but any scoop you make of the mixture will have different amounts of the substances in it.

Mixtures – Homogeneous • A homogeneous mixture is a mixture in which the individual substances are evenly mixed. • In these mixtures, the particles are so small and so well-mixed that they aren’t visible even with most microscopes.

Mixtures – Homogeneous • Some homogenous mixtures are obvious because you know how they’re formed. • Tea is a homogenous mixture. • Tea leaves are mixed with water to form the tea beverage.

Mixtures – Homogeneous • Some homogenous mixtures are less obvious. • Brass is a solid homogenous mixture of copper and zinc. • The copper and zinc are both melted and stirred together, but they do not chemically bond.

Solution – Solvent, solutes, and dissolving • Another name for a homogeneous mixture is a solution. • In a solution, the substance present in the largest amount is called the solvent. • The other substances are called solutes.

Solution – Solvent, solutes, and dissolving • The solutes dissolve into the solvent. • To dissolve means to form a solution by mixing evenly. • Even if the amounts of solute and solvent are different, they are evenly mixed together.

Mixtures - Homogeneous • Think of apple juice. • Two glasses of apple juice from the same container will contain the same substances in the same amounts. • Two different jugs of apple juice, however, might have different amounts of water, sugar, and other substances.

Mixtures - Homogeneous • Apple juice is also definitely a mixture, because physical processes can separate it. • Let the water evaporate, and the rest is left behind. • Let the container of apple juice sit too long without shaking and sometimes the other ingredients can come out of solution.