Matter and Energy What is matter Matter and























- Slides: 41

Matter and Energy • What is matter?

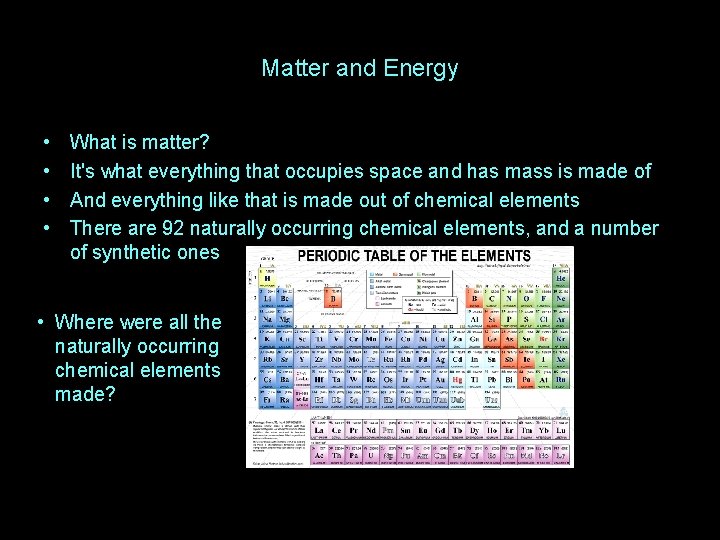
Matter and Energy • • What is matter? It's what everything that occupies space and has mass is made of And everything like that is made out of chemical elements There are 92 naturally occurring chemical elements, and a number of synthetic ones

Matter and Energy • • What is matter? It's what everything that occupies space and has mass is made of And everything like that is made out of chemical elements There are 92 naturally occurring chemical elements, and a number of synthetic ones

Matter and Energy • • What is matter? It's what everything that occupies space and has mass is made of And everything like that is made out of chemical elements There are 92 naturally occurring chemical elements, and a number of synthetic ones

Matter and Energy • • What is matter? It's what everything that occupies space and has mass is made of And everything like that is made out of chemical elements There are 92 naturally occurring chemical elements, and a number of synthetic ones • Where were all the naturally occurring chemical elements made?

Matter and Energy • • What is matter? It's what everything that occupies space and has mass is made of And everything like that is made out of chemical elements There are 92 naturally occurring chemical elements, and a number of synthetic ones: • Where were all the naturally occurring chemical elements made? INSIDE STARS

Matter and Energy • • What is energy? It’s what makes things happen It’s what makes matter move We buy energy every day • What are some forms of energy you’ve bought this week? • So you can see that energy comes in a variety of forms

Matter and Energy • Forms of Energy – Kinetic energy -- energy of motion – Potential energy -- energy stored for later release as kinetic or radiative…there are several types: • gravitational • chemical • electrical • nuclear – Radiative energy -- energy carried by electromagnetic waves

Matter and Energy • The different forms of energy can be converted into one another • Understanding the conversions is essential to understanding astronomy • And this is tied to another fundamental conservation law: Conservation of Energy

Matter and Energy Conservation of Energy • In an isolated system, energy can change form, but the total amount never changes • Anything that happens involves an exchange of energy between material objects and/or the conversion of energy from one form to another. • Here's an example… • Chemical PE (food) KE (lifting weight) gravitational PE (holding weight) KE (weight falling) various KEs (thermal energy, work, sound)

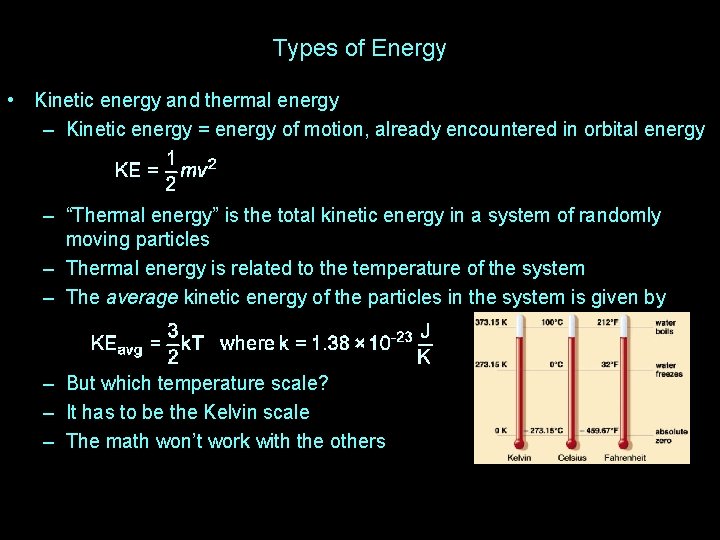
Types of Energy • Kinetic energy and thermal energy – Kinetic energy = energy of motion, already encountered in orbital energy – “Thermal energy” is the total kinetic energy in a system of randomly moving particles – Thermal energy is related to the temperature of the system – The average kinetic energy of the particles in the system is given by – But which temperature scale? – It has to be the Kelvin scale – The math won’t work with the others

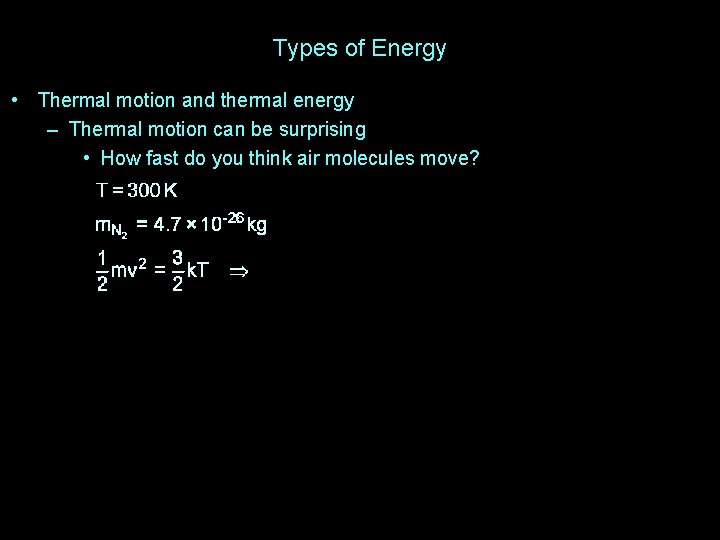
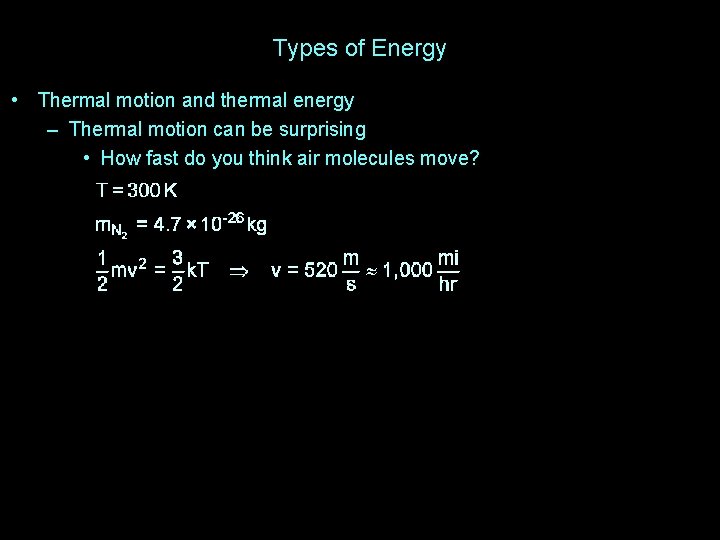
Types of Energy • Thermal motion and thermal energy – Thermal motion can be surprising • How fast do you think air molecules move?

Types of Energy • Thermal motion and thermal energy – Thermal motion can be surprising • How fast do you think air molecules move?

Types of Energy • Thermal motion and thermal energy – Thermal motion can be surprising • How fast do you think air molecules move?

Types of Energy • Thermal motion and thermal energy – Thermal motion can be surprising • How fast do you think air molecules move?

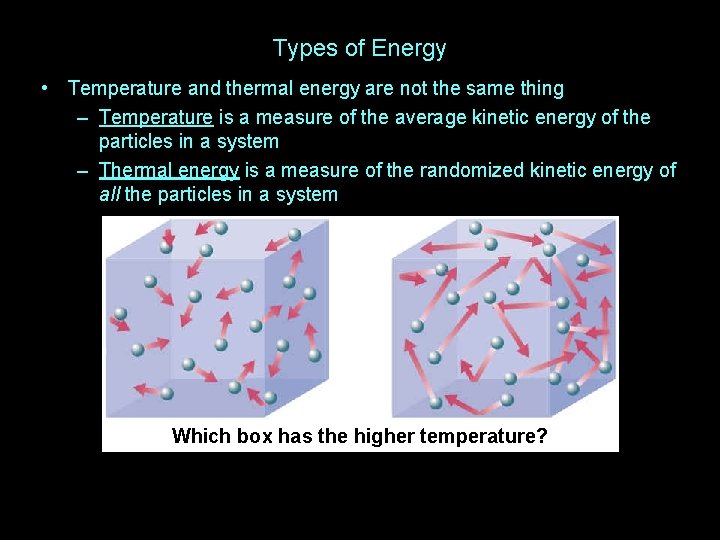
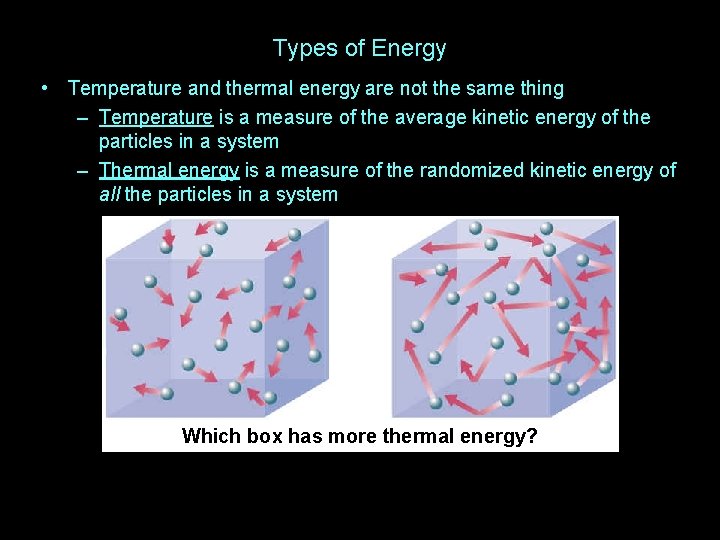
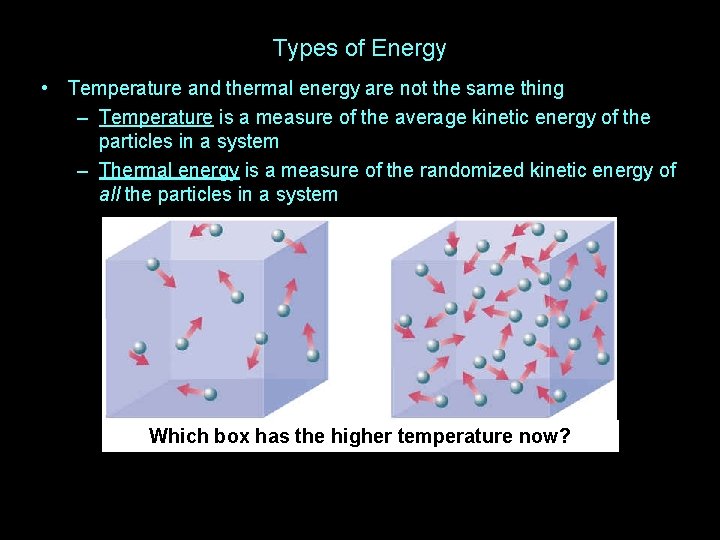
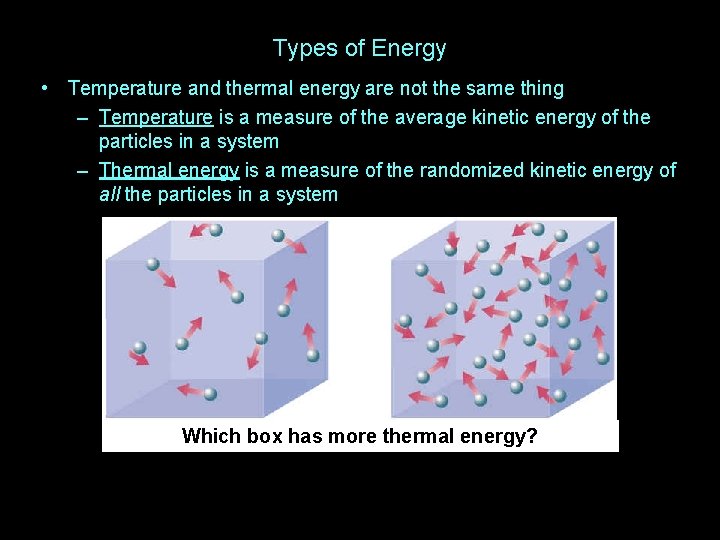
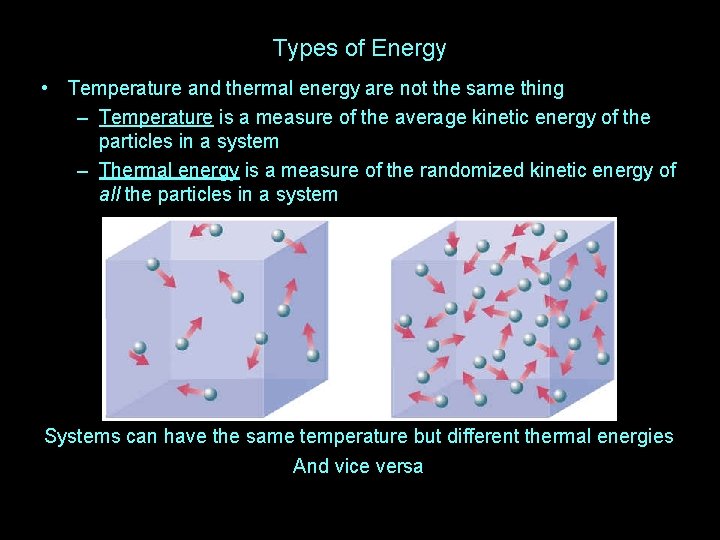
Types of Energy • Temperature and thermal energy are not the same thing

Types of Energy • Temperature and thermal energy are not the same thing – Temperature is a measure of the average kinetic energy of the particles in a system

Types of Energy • Temperature and thermal energy are not the same thing – Temperature is a measure of the average kinetic energy of the particles in a system – Thermal energy is a measure of the randomized kinetic energy of all the particles in a system

Types of Energy • Temperature and thermal energy are not the same thing – Temperature is a measure of the average kinetic energy of the particles in a system – Thermal energy is a measure of the randomized kinetic energy of all the particles in a system Longer arrows mean higher average speed

Types of Energy • Temperature and thermal energy are not the same thing – Temperature is a measure of the average kinetic energy of the particles in a system – Thermal energy is a measure of the randomized kinetic energy of all the particles in a system Which box has the higher temperature?

Types of Energy • Temperature and thermal energy are not the same thing – Temperature is a measure of the average kinetic energy of the particles in a system – Thermal energy is a measure of the randomized kinetic energy of all the particles in a system Which box has more thermal energy?

Types of Energy • Temperature and thermal energy are not the same thing – Temperature is a measure of the average kinetic energy of the particles in a system – Thermal energy is a measure of the randomized kinetic energy of all the particles in a system Which box has the higher temperature now?

Types of Energy • Temperature and thermal energy are not the same thing – Temperature is a measure of the average kinetic energy of the particles in a system – Thermal energy is a measure of the randomized kinetic energy of all the particles in a system Which box has more thermal energy?

Types of Energy • Temperature and thermal energy are not the same thing – Temperature is a measure of the average kinetic energy of the particles in a system – Thermal energy is a measure of the randomized kinetic energy of all the particles in a system Systems can have the same temperature but different thermal energies And vice versa

Types of Energy • Temperature and thermal energy are not the same thing – Temperature is a measure of the average kinetic energy of the particles in a system – Thermal energy is a measure of the randomized kinetic energy of all the particles in a system It is thermal energy, not temperature, that causes burns Think about this kitchen example… The 212ºF oven has less thermal energy than the boiling water

More Types of Energy • Potential energy – Gravitational potential energy, already encountered in orbital energy – Mass-energy

Important Types of Energy for Astronomy • Kinetic energy – energy of motion • Thermal energy – randomized kinetic energy of collection of particles This is the average KE of the particles • Gravitational potential energy – energy due to position in a gravity field • Mass-energy – energy equivalent of mass

• • How much mass do you think is equivalent to the amount of energy released by metabolizing a ~2 ounce candy bar – 1 x 106 J? – merely half a billionth of an ounce How much energy is contained in 1 kg of mass? – 9 x 1016 J, the equivalent of an 18 megaton nuclear bomb, 1200 times the bomb that wiped out Hiroshima in 1945 – 1600 X more than is released by fissioning 1 kg of uranium!

The Material World • What does energy do? . . . …it moves matter, and that’s what we’ll talk about now • What is matter made of? . . . …the chemical elements • There are 92 naturally occurring chemical elements • So whey are there many more substances than that? . . . …because atoms combine to form molecules and compounds… • …with very different properties from the elements they are made of: H 2 gas O 2 gas S solid H 2 O liquid H 2 SO 4 liquid

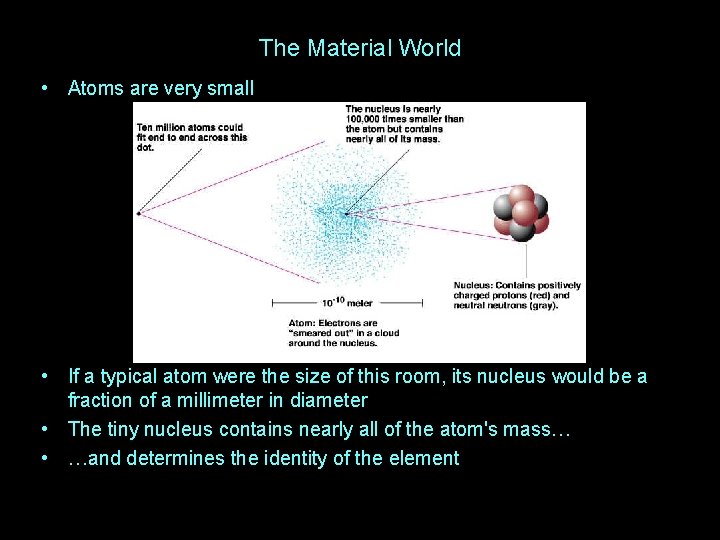
The Material World • Atoms are very small • If a typical atom were the size of this room, its nucleus would be a fraction of a millimeter in diameter • The tiny nucleus contains nearly all of the atom's mass… • …and determines the identity of the element

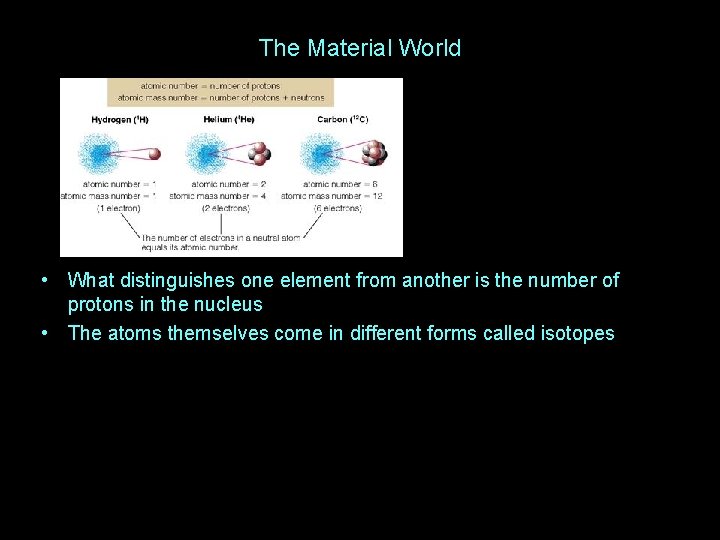
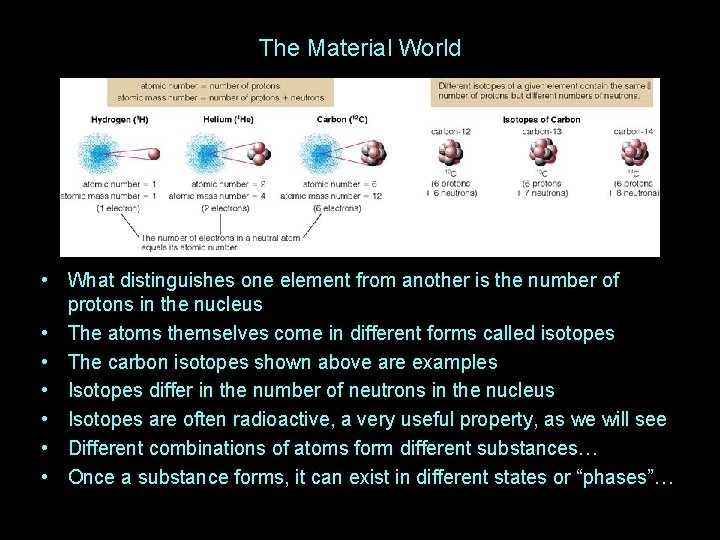
The Material World • What distinguishes one element from another is the number of protons in the nucleus • The atoms themselves come in different forms called isotopes

The Material World • What distinguishes one element from another is the number of protons in the nucleus • The atoms themselves come in different forms called isotopes • The carbon isotopes shown above are examples • Isotopes differ in the number of neutrons in the nucleus • Isotopes are often radioactive, a very useful property, as we will see • Different combinations of atoms form different substances… • Once a substance forms, it can exist in different states or “phases”…

The Material World •

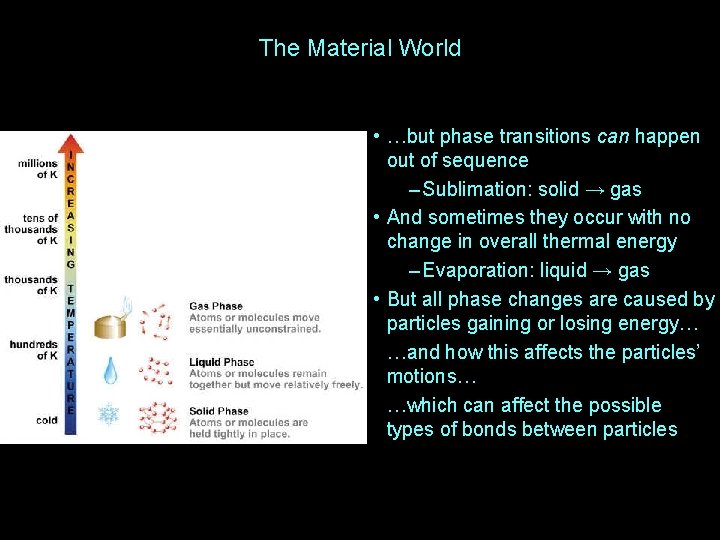
The Material World • …but phase transitions can happen out of sequence – Sublimation: solid → gas • And sometimes they occur with no change in overall thermal energy – Evaporation: liquid → gas • But all phase changes are caused by particles gaining or losing energy… …and how this affects the particles’ motions… …which can affect the possible types of bonds between particles

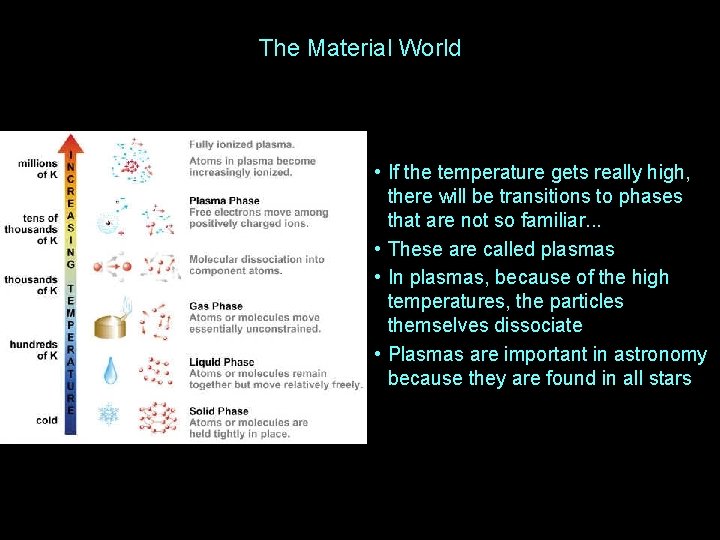
The Material World • If the temperature gets really high, there will be transitions to phases that are not so familiar. . . • These are called plasmas • In plasmas, because of the high temperatures, the particles themselves dissociate • Plasmas are important in astronomy because they are found in all stars

Do you understand phase changes? • Suppose that a chunk of an unknown solid substance was found on an alien world in which the bonds between atoms were unbreakable. Would phase changes be possible for this substance? A. Yes B. No

Do you understand phase changes? • Suppose that a chunk of an unknown solid substance was found on an alien world in which the bonds between atoms were unbreakable. Would phase changes be possible for this substance? A. Yes B. No, because if the bonds can’t be broken, the phase can’t change

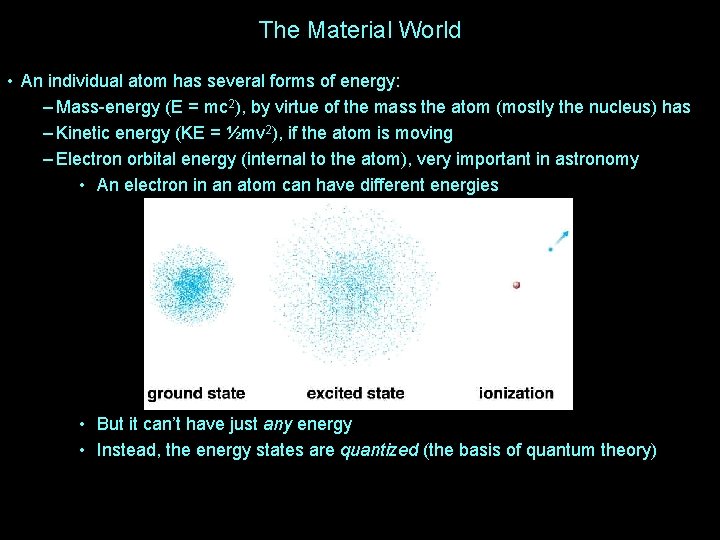
The Material World • An individual atom has several forms of energy: – Mass-energy (E = mc 2), by virtue of the mass the atom (mostly the nucleus) has – Kinetic energy (KE = ½mv 2), if the atom is moving – Electron orbital energy (internal to the atom), very important in astronomy • An electron in an atom can have different energies • But it can’t have just any energy • Instead, the energy states are quantized (the basis of quantum theory)

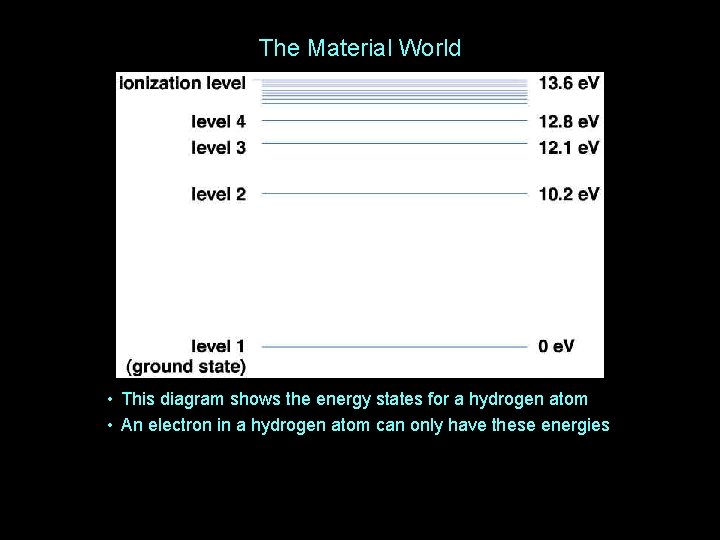
The Material World • This diagram shows the energy states for a hydrogen atom • An electron in a hydrogen atom can only have these energies

The Material World • The electron can move up and down (or “transition”) between these energy states, gaining and losing energy in the process • But transitions only happen if the energy gained or lost exactly equals the difference between energy states • And this leads to one of the most important techniques used in astronomy

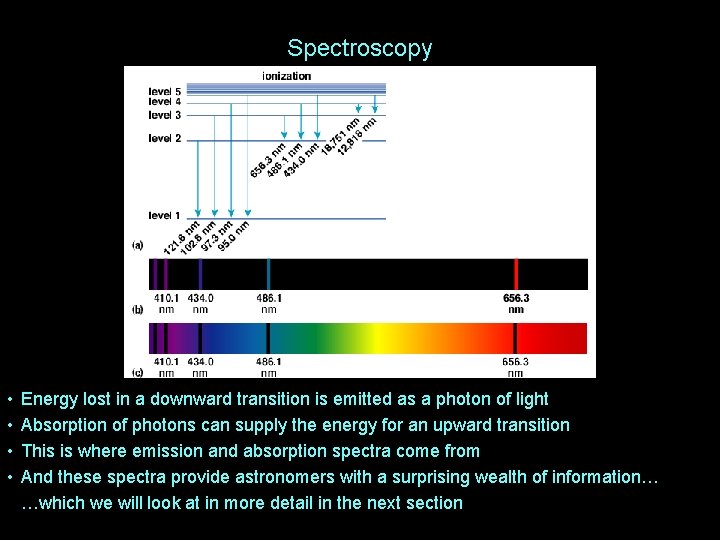
Spectroscopy • • Energy lost in a downward transition is emitted as a photon of light Absorption of photons can supply the energy for an upward transition This is where emission and absorption spectra come from And these spectra provide astronomers with a surprising wealth of information… …which we will look at in more detail in the next section
 Flow energy review
Flow energy review Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Gray matter in the brain
Gray matter in the brain Primary taste cortex
Primary taste cortex Gray matter and white matter
Gray matter and white matter Gray matter vs white matter
Gray matter vs white matter Lesson 3 energy and matter in ecosystems answer key
Lesson 3 energy and matter in ecosystems answer key Section 16.1 thermal energy and matter
Section 16.1 thermal energy and matter Section 1 matter and thermal energy
Section 1 matter and thermal energy Science matter
Science matter Matter energy and measurement
Matter energy and measurement Dark matter and dark energy ppt
Dark matter and dark energy ppt Unit 2 matter and energy
Unit 2 matter and energy List the secondary consumers
List the secondary consumers The science duo physical and chemical changes
The science duo physical and chemical changes States of matter concept map
States of matter concept map States of matter foldable
States of matter foldable Venn diagram mechanical and electromagnetic waves
Venn diagram mechanical and electromagnetic waves Kesler science
Kesler science Which energy
Which energy Section 1 composition of matter
Section 1 composition of matter Section 1 composition of matter
Section 1 composition of matter Chapter 2 section 1 classifying matter answer key
Chapter 2 section 1 classifying matter answer key Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Describing energy section 2 answers
Describing energy section 2 answers Primary energy and secondary energy
Primary energy and secondary energy What is commercial energy source
What is commercial energy source Helmholtz free energy
Helmholtz free energy Renewable energy and energy efficiency partnership
Renewable energy and energy efficiency partnership Potential energy examples pictures
Potential energy examples pictures The change in mechanical energy
The change in mechanical energy Mechanical advantage
Mechanical advantage Potential energy units
Potential energy units Eroei
Eroei Mechanical wave energy
Mechanical wave energy Waves transfer energy without transferring matter
Waves transfer energy without transferring matter Thermal energy states of matter
Thermal energy states of matter A rhythmic movement that carries energy
A rhythmic movement that carries energy Flow of energy vs flow of matter
Flow of energy vs flow of matter A rhythmic disturbance that transfers energy
A rhythmic disturbance that transfers energy Thermal energy in states of matter
Thermal energy in states of matter