Matter and Energy When Matter and energy interact















- Slides: 32

Matter and Energy When Matter and energy interact changes in matter occur.

Energy Definition: the ability to do work Is NOT matter Is measured in the unit Joules (J) Can be POTENTIAL or KINETIC

Potential Energy that is stored Something has the “potential” to do some kind of work Example: the child at the top of the slide has potential energy

Kinetic Energy of motion Example: the child going down the slide now has kinetic energy

Different types of energy… Light is waves, visible or invisible Electrical involves moving electrons Heat movement of molecules Chemical is contained in foods Nuclear responsible for the sun Sound waves/vibrations of molecules Mechanical involves moving objects Magnetic opposing poles

Law of Conservation of Energy, like matter, is neither created nor destroyed, rather it is converted.

Summary: What will we study in this unit? What is heat? How is it different from temperature? How does energy relate to chemical reactions? How energy relates to phase changes?

Understanding Heat Flow Heat (q) is defined as the energy that transfers from one object to another. Heat flows from warm cool. What will happen if the two objects are touching?

Heat Energy vs. Temperature is measure of the heat flow. Temperature is a measure of the average kinetic energy of the particles in matter.

Heat Energy and Changes in Matter In virtually all changes in matter, energy is released or absorbed. System vs. Surroundings

Examples

Exothermic Reactions (Changes) Exothermic reactions RELEASE ENERGY Has a Negative q value. Heat is a product.

Endothermic Reactions (Changes) Endothermic reactions ABSORB ENERGY Has a Positive q value because heat is entering the system. Heat is a reactant.

Activation Energy Sometimes reactions can’t occur on their own They need a little input of energy to get it started. This energy is called ACTIVATION ENERGY.

Energy and Phase Changes Energy of particles of matter relates to the phase or state of matter (solid, liquid, or gas) Changes in energy then result in changes of matter.

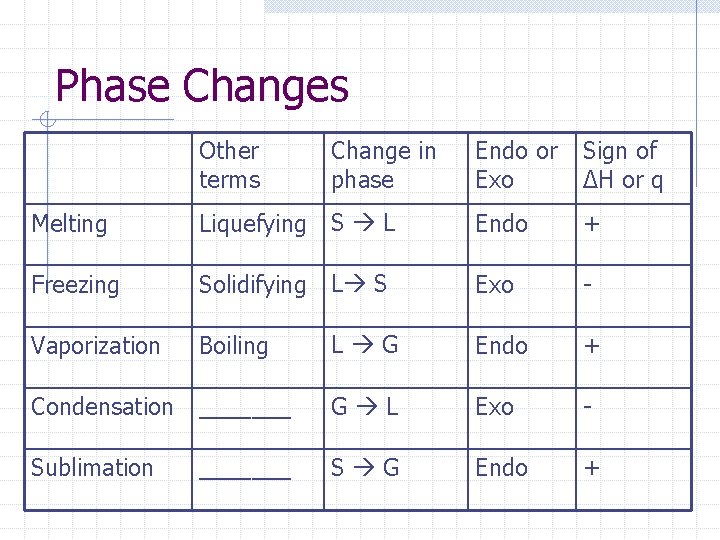
Phase Changes Other terms Change in phase Endo or Exo Sign of ΔH or q Endo + Melting Liquefying S L Freezing Solidifying L S Exo - Vaporization Boiling L G Endo + Condensation _______ G L Exo - Sublimation _______ S G Endo +

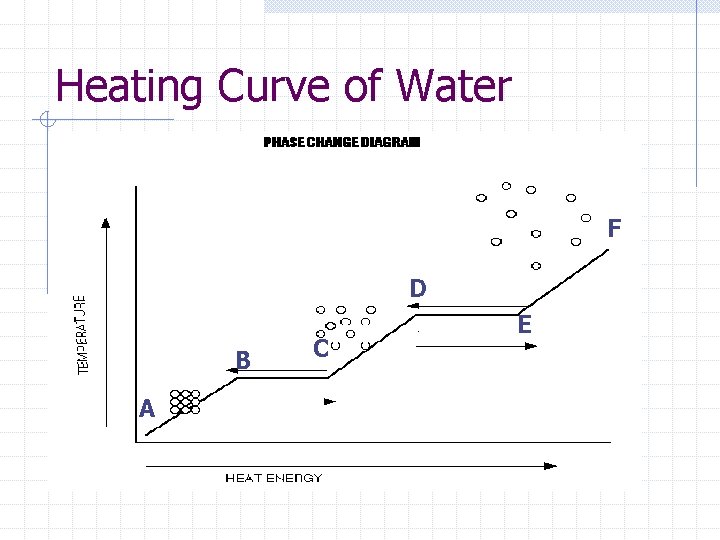
Heating Curve of Water F D B A C E

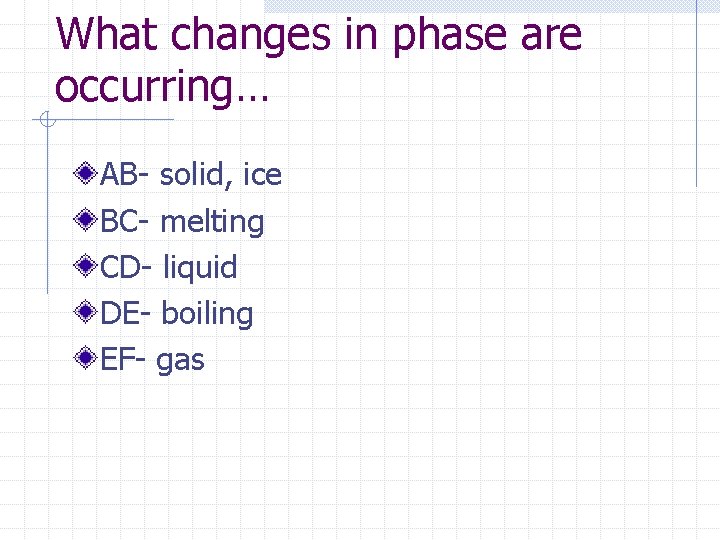
What changes in phase are occurring… AB- solid, ice BC- melting CD- liquid DE- boiling EF- gas

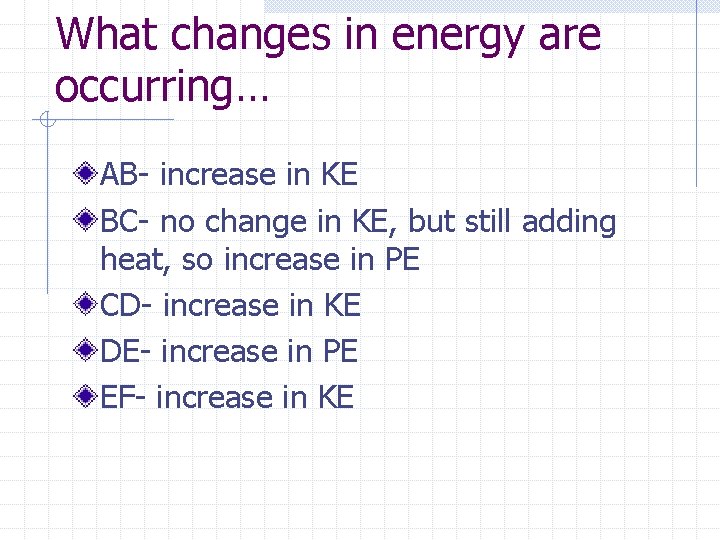
What changes in energy are occurring… AB- increase in KE BC- no change in KE, but still adding heat, so increase in PE CD- increase in KE DE- increase in PE EF- increase in KE

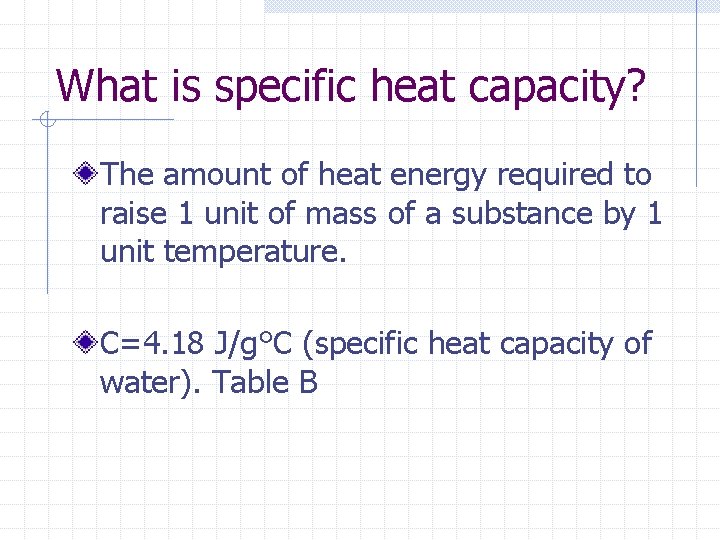
What is specific heat capacity? The amount of heat energy required to raise 1 unit of mass of a substance by 1 unit temperature. C=4. 18 J/g°C (specific heat capacity of water). Table B

Heat of Fusion Heat of fusion (Hf) = amount of heat energy absorbed or released when melting or freezing. See Reference Table B for values Ex: Hf H 2 O = 334 J/g

Heat of Vaporization Heat of vaporization = heat absorbed or released when vaporizing or condensing. See Reference Tables for values. Ex: HVH 2 O = 2260 J/g

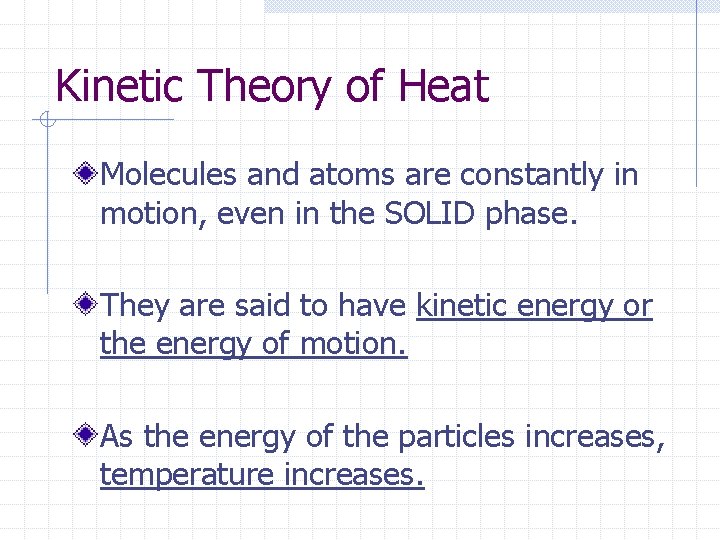
Kinetic Theory of Heat Molecules and atoms are constantly in motion, even in the SOLID phase. They are said to have kinetic energy or the energy of motion. As the energy of the particles increases, temperature increases.

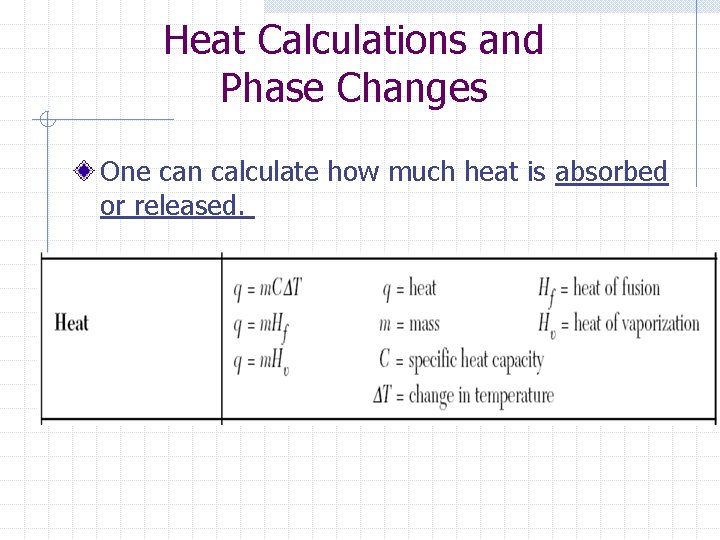
Heat Calculations and Phase Changes One can calculate how much heat is absorbed or released.

Potential energy If the problem says… Melt/freeze Vaporize condense At 0°C (melting/freezing point) At 100°C (boiling/condensing point) Use Q = m. Hf (melting/freezing) or Q = m. Hv (vaporization/condensation)

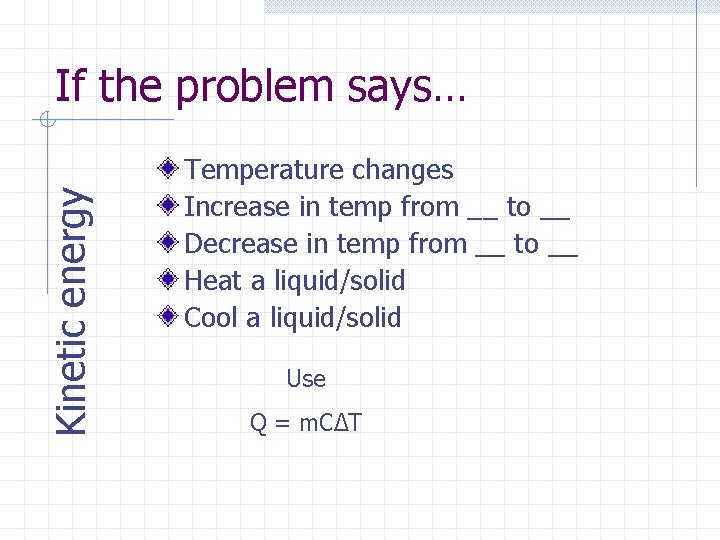
Kinetic energy If the problem says… Temperature changes Increase in temp from __ to __ Decrease in temp from __ to __ Heat a liquid/solid Cool a liquid/solid Use Q = m. CΔT

Examples How much heat is needed to melt 10. 5 grams of ice at 0°C? Use Q = m. Hf Q = (10. 5 g) (334 J/g) Q= 3507 J

Examples What mass of liquid water can be vaporized if 680 J of heat energy is added at 100°C? Use Q = m. Hv 680 J = (m) (2260 J/g) m= 0. 30 g

Examples How much energy is needed to increase the temperature of 5. 0 grams of water from 0°C to 10°C? Use Q = m. CΔT Q = (5. 0 g) (4. 18 J/g°C)(10 -0) Q= 209 J

Examples What is the mass of water that can be increased in temperature by 15°C by the addition of 800 J? Use Q = m. CΔT 800 J = (m) (4. 18 J/g°C)(15°C) m= 12. 8 g

Calorimetry Used to measure amount of heat released or absorbed during a chemical/physical change that occurs in water solution. “calorimeter” is used to measure the change in temperature of water surrounding a reaction.

Cheap Calorimeter- insulation