MATTER AND ENERGY MATTER Matter is anything that














- Slides: 22

MATTER AND ENERGY

MATTER Matter is anything that has mass and volume Two forms of matter Pure Substances Mixtures

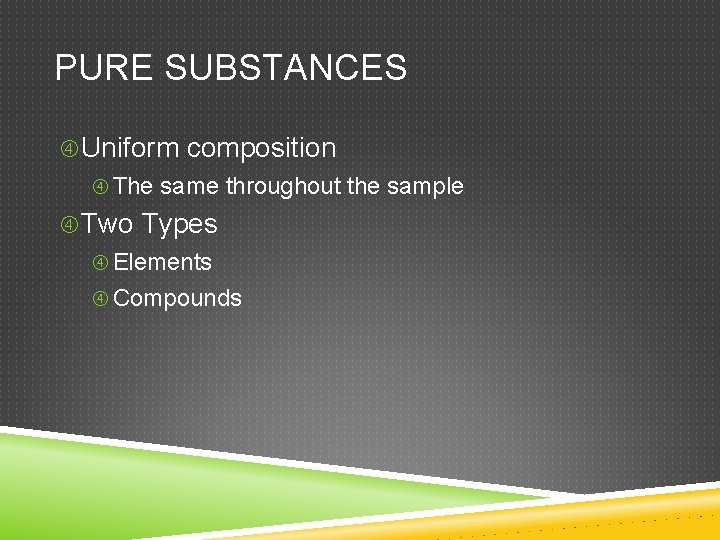
PURE SUBSTANCES Uniform composition The same throughout the sample Two Types Elements Compounds

ELEMENTS Simplest form of matter Cannot breakdown Smallest part called atom Represented using a capital letter or capital letter and lower case letter

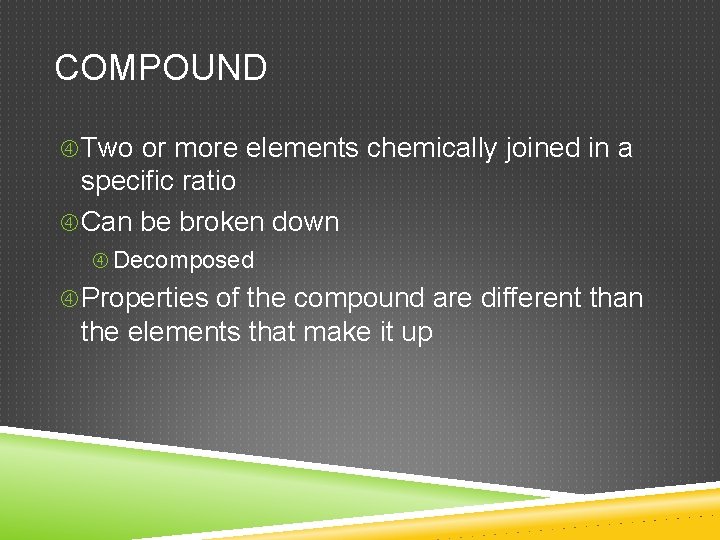
COMPOUND Two or more elements chemically joined in a specific ratio Can be broken down Decomposed Properties of the compound are different than the elements that make it up

MIXTURE Two or more substances physically joined in any ratio Keep the properties of the components of the mixture Two types Heterogeneous Homogeneous

Heterogeneous Visible difference between components (parts) Homogeneous No visible differences between components (parts) Called a solution Represented using (aq) aqueous

PROPERTIES OF MATTER Physical Properties that can be observed without changing the substance Chemical Properties that show a substance reacts (changes)

ENERGY Energy is the driving force behind change Cannot be created or destroyed Does change its form Sound Chemical Radiant (light) Electrical Atomic (nuclear) Mechanical Thermal (heat)

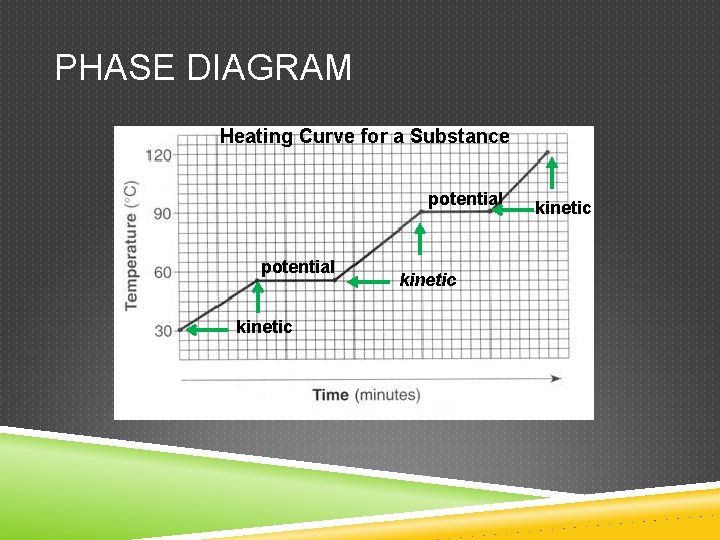
Two types of energy Kinetic Energy of motion Potential Stored energy

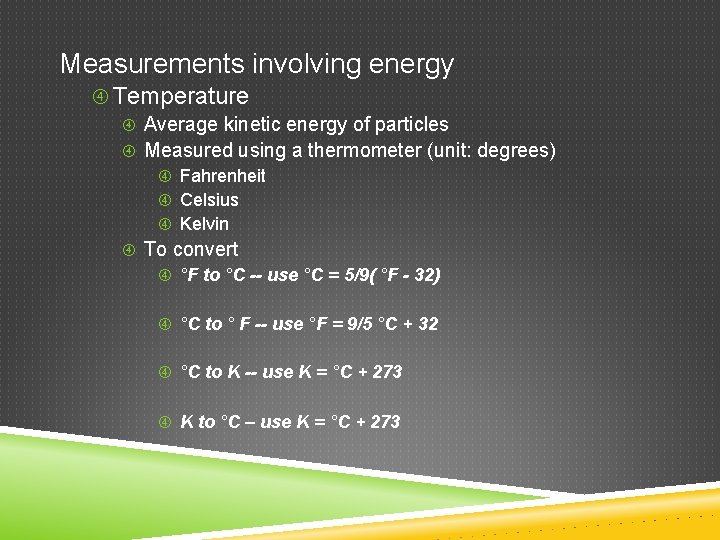
Measurements involving energy Temperature Average kinetic energy of particles Measured using a thermometer (unit: degrees) Fahrenheit Celsius Kelvin To convert °F to °C -- use °C = 5/9( °F - 32) °C to ° F -- use °F = 9/5 °C + 32 °C to K -- use K = °C + 273 K to °C – use K = °C + 273

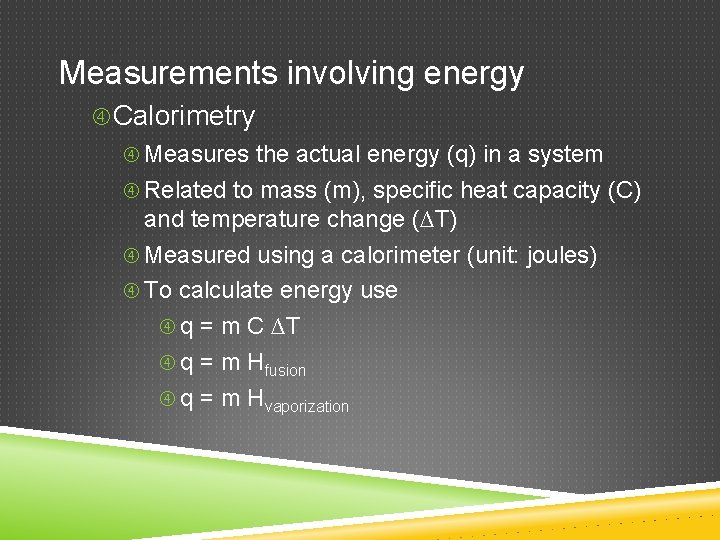
Measurements involving energy Calorimetry Measures the actual energy (q) in a system Related to mass (m), specific heat capacity (C) and temperature change (∆T) Measured using a calorimeter (unit: joules) To calculate energy use q = m C ∆T q = m Hfusion q = m Hvaporization

Cwater = 4. 18 J/g °C Hfus = 334 J/g Hvap = 2260 J/g How many joules are required to heat 40 g water at 30°C to 70°C? q = m C ∆T q = 40 g x 4. 18 J/g°C x 40°C q = 6688 J 5000 J were added to 30 g water at 25°C. What is the new temperature? q = m C ∆T 5000 J = 30 g x 4. 18 J/g°C x ∆T 5000 = 125. 4 x ∆T ∆T = 39. 9 ~ 40 T new = 25 + 40 T new = 65°C How many joules are needed to melt 100 g ice at 0°C q = m Hfus q = 100 g x 334 J/g q = 33400 J

PHASES OF MATTER Solids Liquids Gases

SOLIDS Matter that has specific shape and specific volume Atoms closely packed together o Cannot be compressed

LIQUIDS Matter that has a specific volume but takes the shape of the container Atoms are close but have some space between them o Cannot be compressed o Can be poured

GASES Matter that takes the shape and volume of the container Atoms have free space between them o Compressible o Can be poured

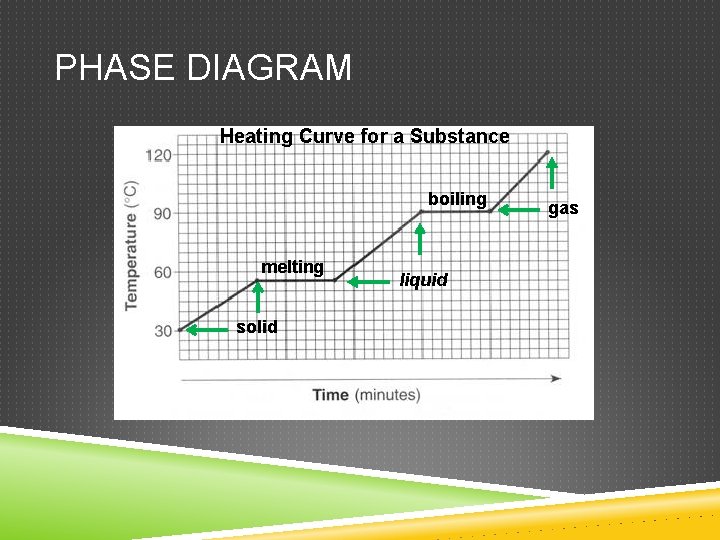
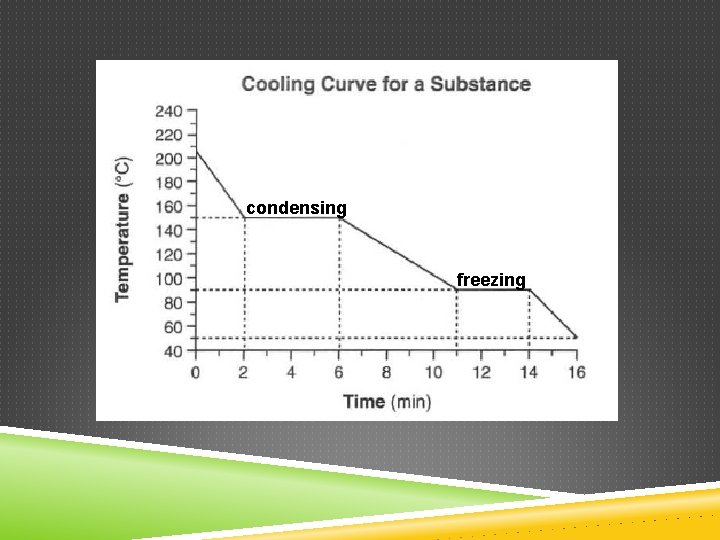
PHASE CHANGES If energy is added… Melting o Solid to liquid Boiling o Liquid to gas Sublimation o Solid to gas

PHASE CHANGES If energy is removed… Freezing o Liquid to solid Condensing o Gas to liquid Deposition o Gas to solid

PHASE DIAGRAM Heating Curve for a Substance boiling melting solid liquid gas

PHASE DIAGRAM Heating Curve for a Substance potential kinetic

condensing freezing