Energy and States of Matter Energy 1 Energy

















- Slides: 69

Energy and States of Matter Energy 1

Energy n Makes objects move. n Makes things stop. n Is needed to “do work. ” 2

Work is done when § You go up stairs. § You play soccer. § You lift a bag of groceries. § You ride a bicycle. § You breathe. § Your heart pumps blood. § Water goes over a dam. 3

Potential Energy § § § Potential energy is energy that is stored for use at a later time. Examples are: Water behind a dam A compressed spring Chemical bonds in gasoline, coal, or food 4

Kinetic Energy Kinetic energy is the energy of motion. Examples are: § Hammering a nail § Water flowing over a dam § Working out § Burning gasoline 5

Learning Check Identify the energy as 1) potential or 2) kinetic A. Roller blading. B. A peanut butter and jelly sandwich. C. Mowing the lawn. D. Gasoline in the gas tank. 6

Solution Identify the energy as 1) potential or 2) kinetic A. Roller blading. (2 kinetic) B. A peanut butter and jelly sandwich. (1 potential) C. Mowing the lawn. (2 kinetic) D. Gasoline in the gas tank. (1 potential) 7

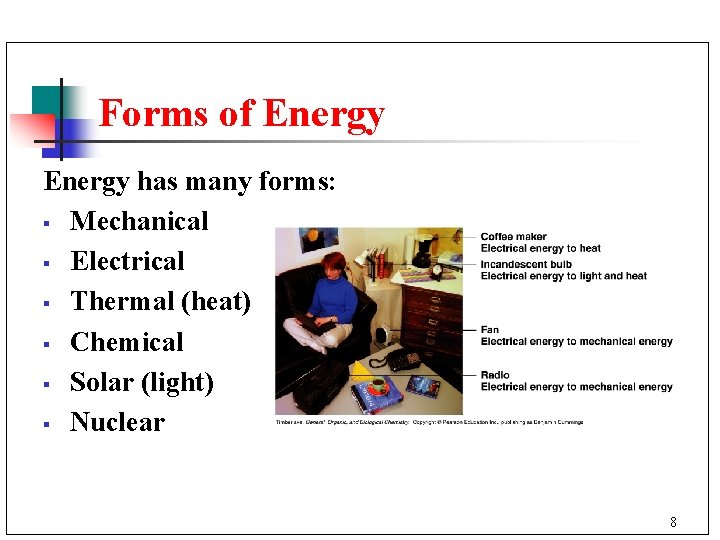
Forms of Energy has many forms: § Mechanical § Electrical § Thermal (heat) § Chemical § Solar (light) § Nuclear 8

Heat § § § Heat energy flows from a warmer object to a colder object. The colder object gains kinetic energy when it is heated. During heat flow, the loss of heat by a warmer object is equal to the heat gained by the colder object. 9

Some Equalities for Heat § Heat is measured in calories or joules. 1 kilocalorie (kcal) = 1000 calories (cal) 1 calorie = 4. 18 Joules (J) 1 k. J = 1000 J 10

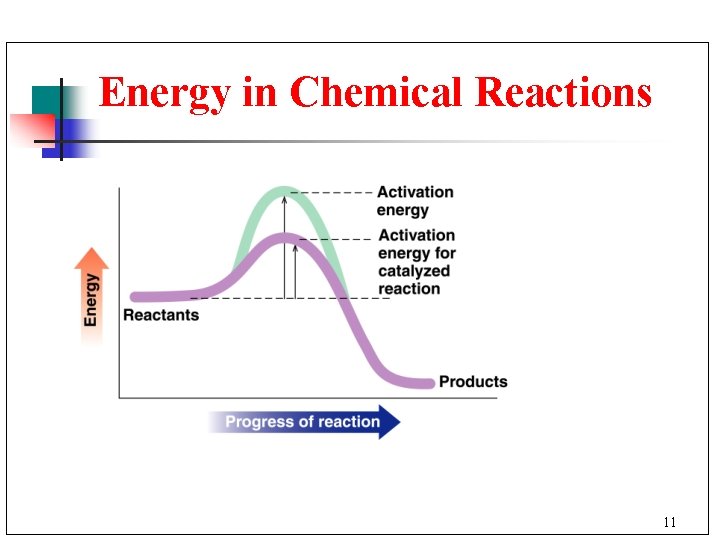
Energy in Chemical Reactions 11

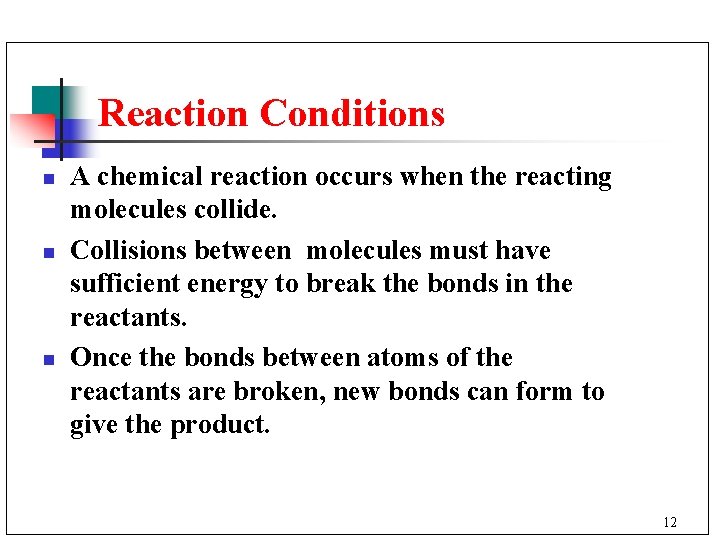
Reaction Conditions n n n A chemical reaction occurs when the reacting molecules collide. Collisions between molecules must have sufficient energy to break the bonds in the reactants. Once the bonds between atoms of the reactants are broken, new bonds can form to give the product. 12

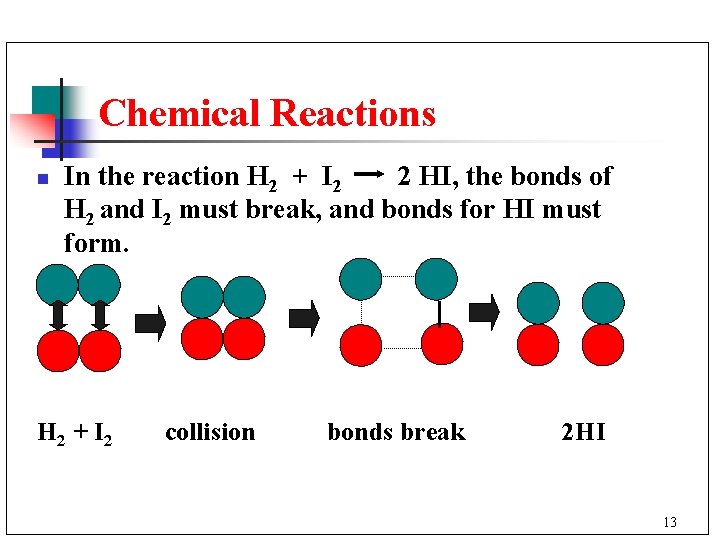
Chemical Reactions n In the reaction H 2 + I 2 2 HI, the bonds of H 2 and I 2 must break, and bonds for HI must form. H 2 + I 2 collision bonds break 2 HI 13

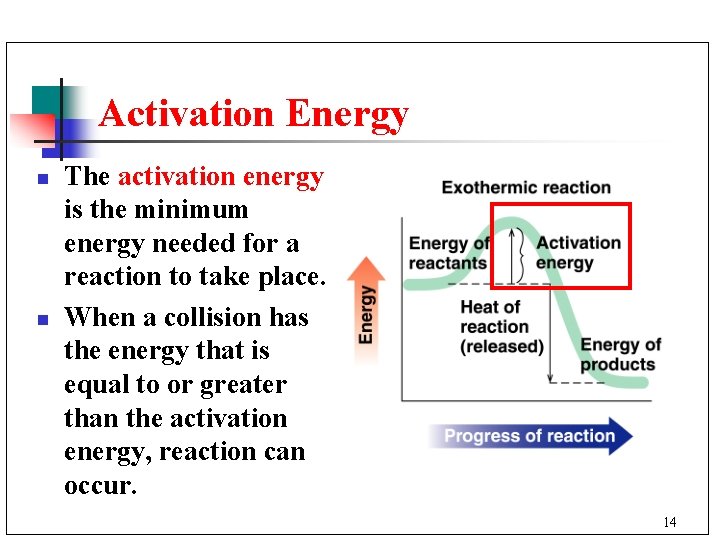
Activation Energy n n The activation energy is the minimum energy needed for a reaction to take place. When a collision has the energy that is equal to or greater than the activation energy, reaction can occur. 14

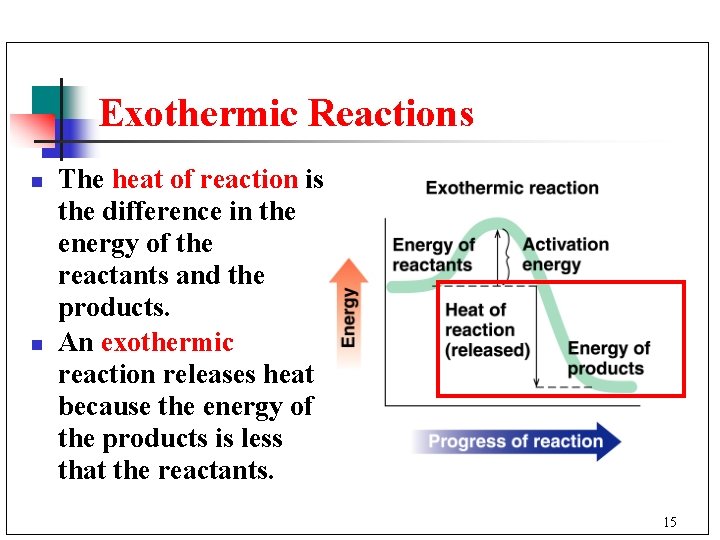
Exothermic Reactions n n The heat of reaction is the difference in the energy of the reactants and the products. An exothermic reaction releases heat because the energy of the products is less that the reactants. 15

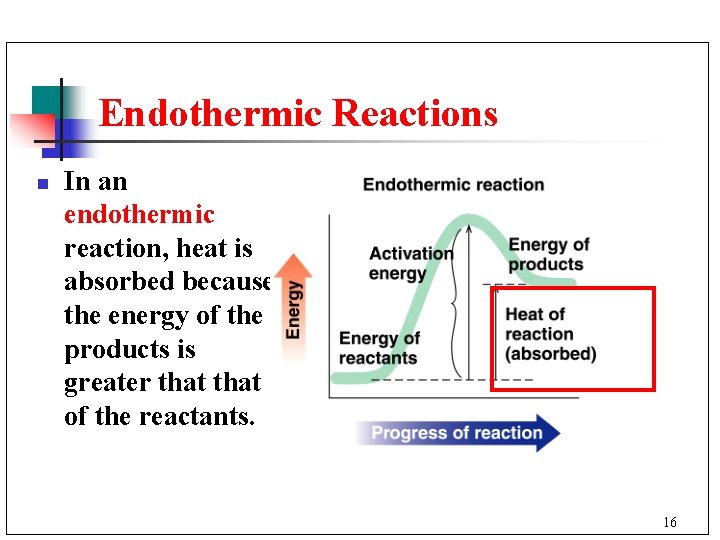
Endothermic Reactions n In an endothermic reaction, heat is absorbed because the energy of the products is greater that of the reactants. 16

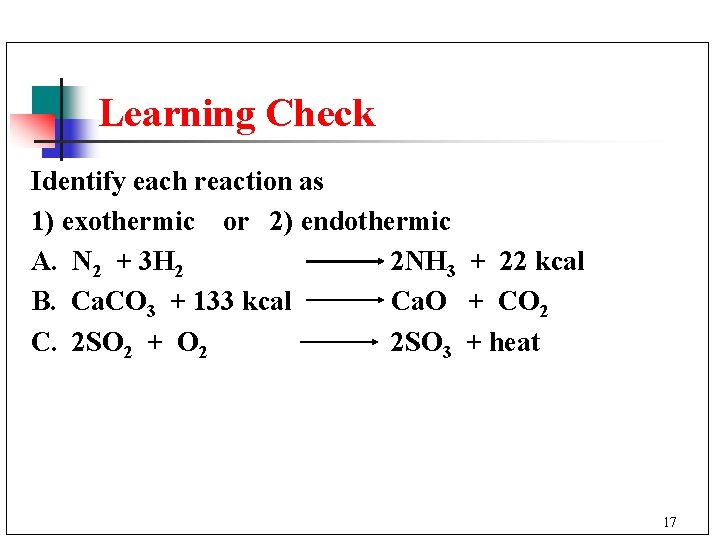
Learning Check Identify each reaction as 1) exothermic or 2) endothermic A. N 2 + 3 H 2 2 NH 3 + 22 kcal B. Ca. CO 3 + 133 kcal Ca. O + CO 2 C. 2 SO 2 + O 2 2 SO 3 + heat 17

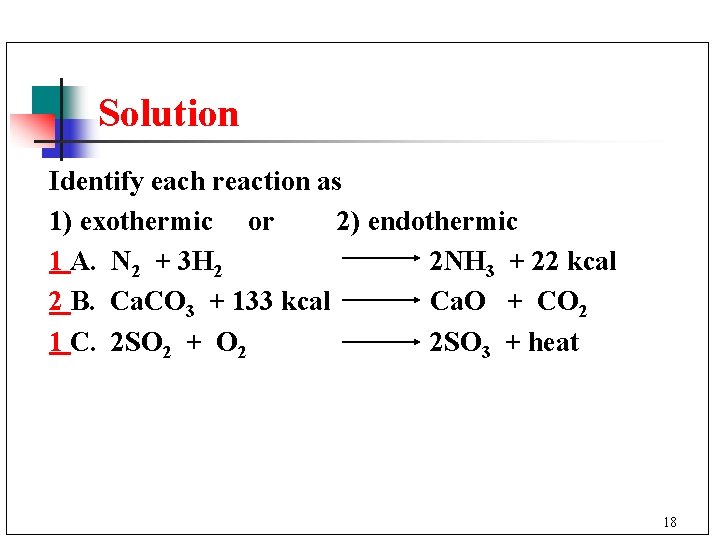
Solution Identify each reaction as 1) exothermic or 2) endothermic 1 A. N 2 + 3 H 2 2 NH 3 + 22 kcal 2 B. Ca. CO 3 + 133 kcal Ca. O + CO 2 1 C. 2 SO 2 + O 2 2 SO 3 + heat 18

Specific Heat Specific heat is the amount of heat (calories or Joules) that raises the temperature of 1 g of a substance by 1°C. 19

Learning Check A. A substance with a large specific heat 1) heats up quickly 2) heats up slowly B. When ocean water cools, the surrounding air 1) cools 2) warms 3) stays the same C. Sand in the desert is hot in the day and cool at night. Sand must have a 1) high specific heat 2) low specific heat 20

Solution A. A substance with a large specific heat 2) heats up slowly B. When ocean water cools, the surrounding air 2) warms C. Sand in the desert is hot in the day and cool at night. Sand must have a 2) low specific heat 21

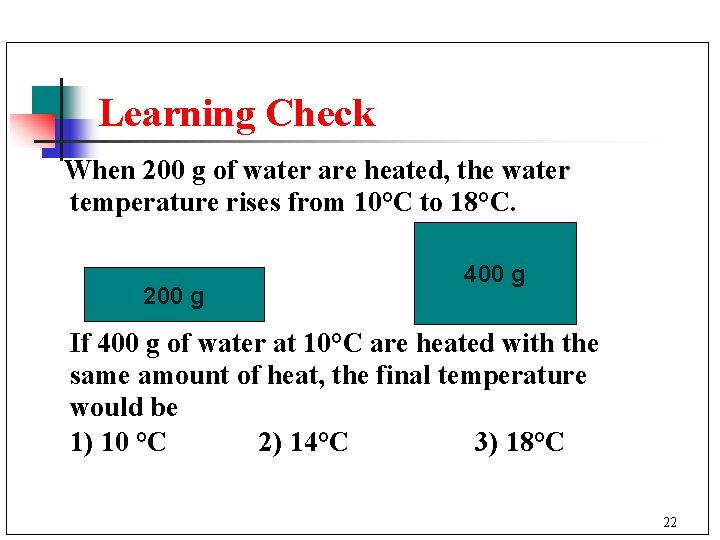
Learning Check When 200 g of water are heated, the water temperature rises from 10°C to 18°C. 200 g 400 g If 400 g of water at 10°C are heated with the same amount of heat, the final temperature would be 1) 10 °C 2) 14°C 3) 18°C 22

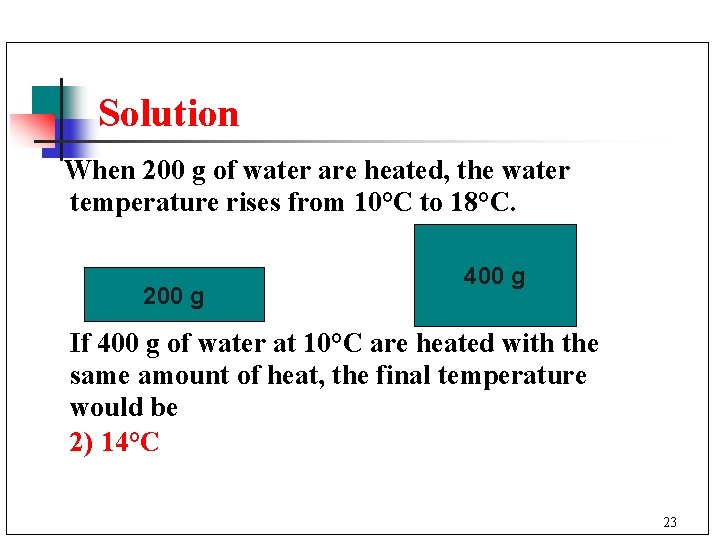
Solution When 200 g of water are heated, the water temperature rises from 10°C to 18°C. 200 g 400 g If 400 g of water at 10°C are heated with the same amount of heat, the final temperature would be 2) 14°C 23

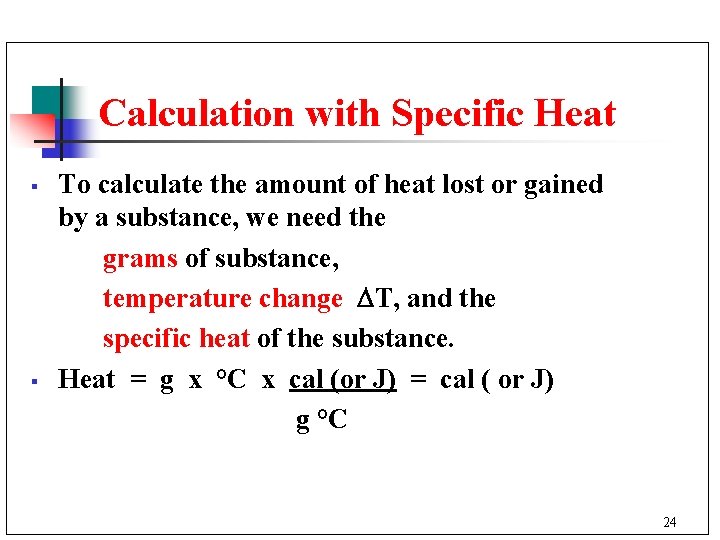
Calculation with Specific Heat § § To calculate the amount of heat lost or gained by a substance, we need the grams of substance, temperature change T, and the specific heat of the substance. Heat = g x °C x cal (or J) = cal ( or J) g °C 24

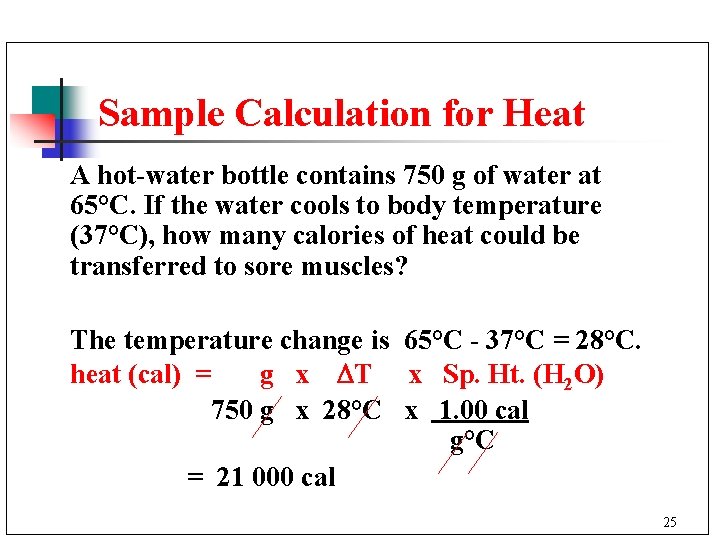
Sample Calculation for Heat A hot-water bottle contains 750 g of water at 65°C. If the water cools to body temperature (37°C), how many calories of heat could be transferred to sore muscles? The temperature change is 65°C - 37°C = 28°C. heat (cal) = g x T x Sp. Ht. (H 2 O) 750 g x 28°C x 1. 00 cal g°C = 21 000 cal 25

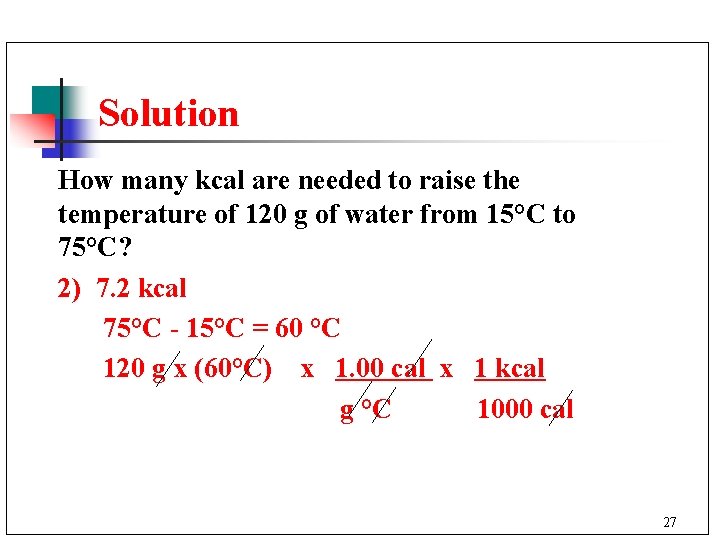
Learning Check How many kcal are needed to raise the temperature of 120 g of water from 15°C to 75°C? 1) 1. 8 kcal 2) 7. 2 kcal 3) 9. 0 kcal 26

Solution How many kcal are needed to raise the temperature of 120 g of water from 15°C to 75°C? 2) 7. 2 kcal 75°C - 15°C = 60 °C 120 g x (60°C) x 1. 00 cal x 1 kcal g °C 1000 cal 27

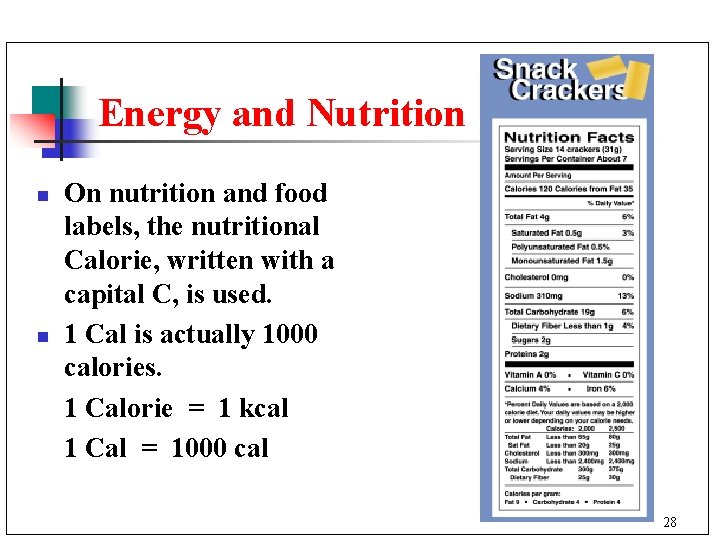
Energy and Nutrition n n On nutrition and food labels, the nutritional Calorie, written with a capital C, is used. 1 Cal is actually 1000 calories. 1 Calorie = 1 kcal 1 Cal = 1000 cal 28

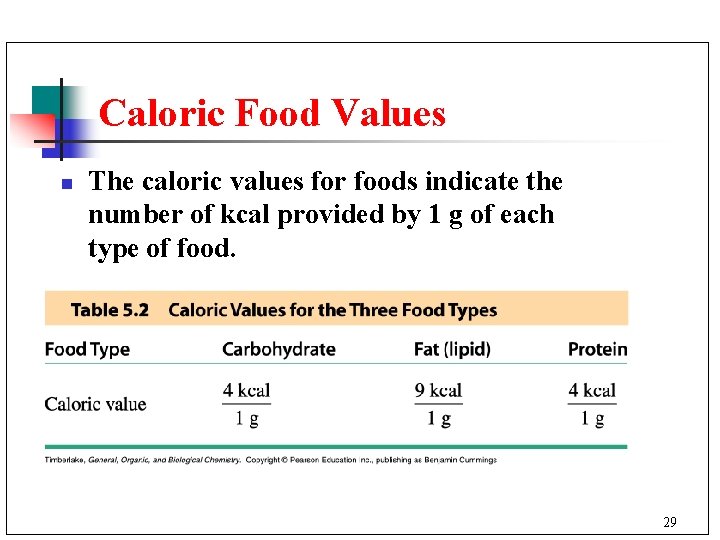
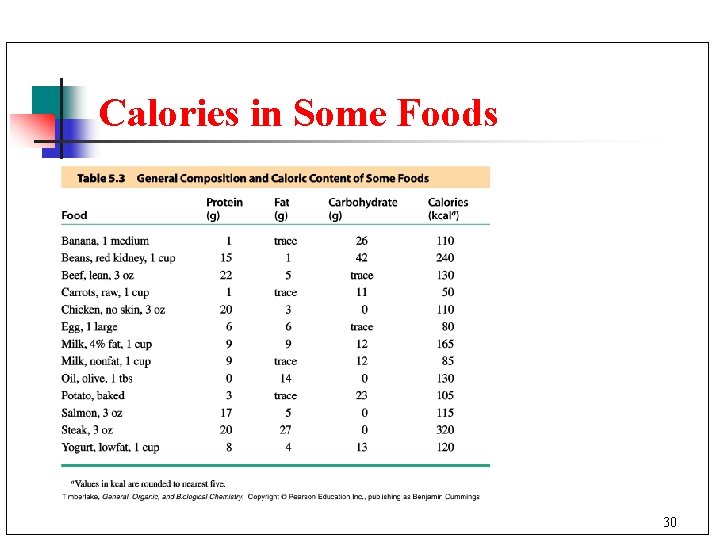
Caloric Food Values n The caloric values for foods indicate the number of kcal provided by 1 g of each type of food. 29

Calories in Some Foods 30

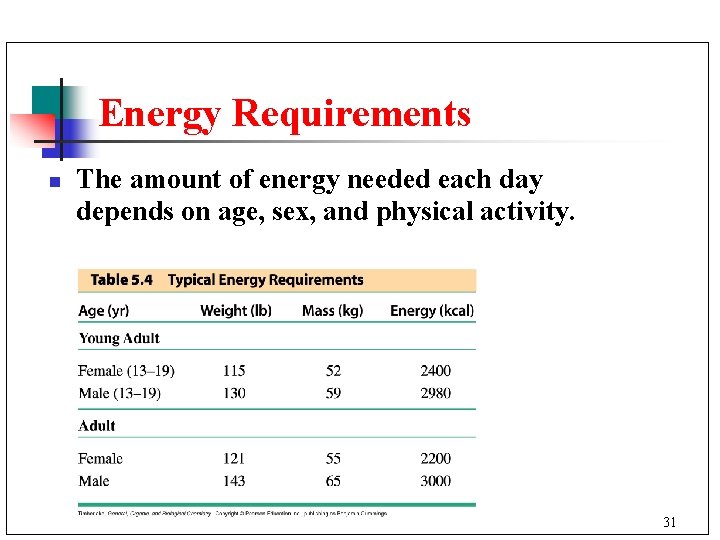
Energy Requirements n The amount of energy needed each day depends on age, sex, and physical activity. 31

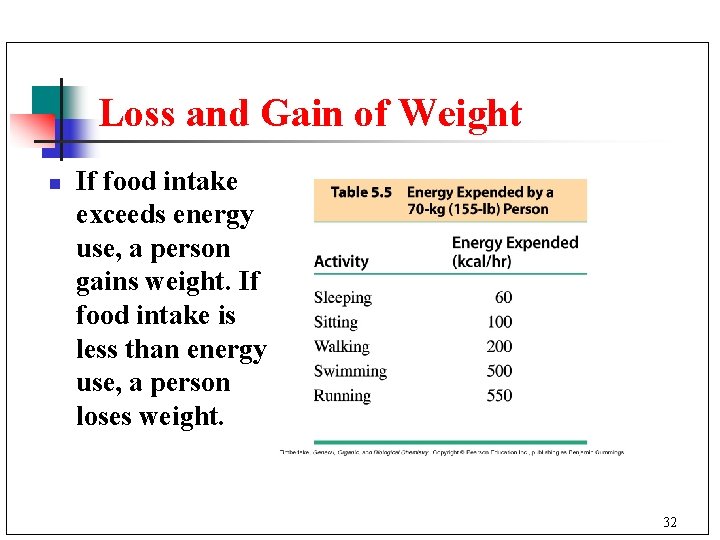
Loss and Gain of Weight n If food intake exceeds energy use, a person gains weight. If food intake is less than energy use, a person loses weight. 32

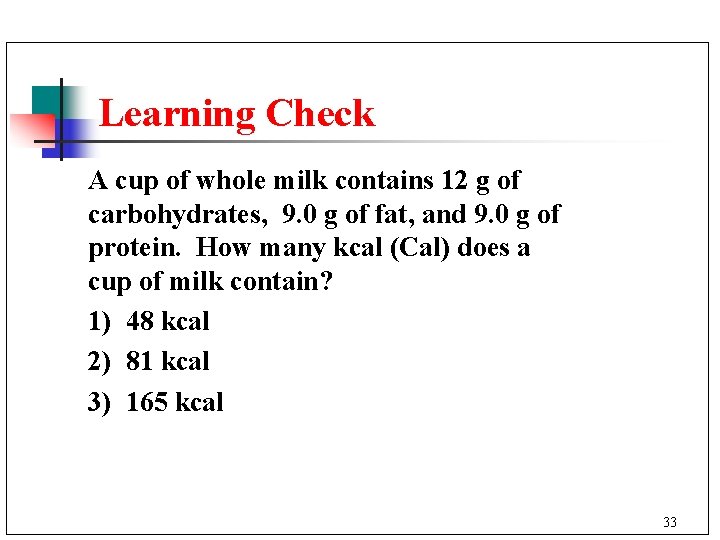
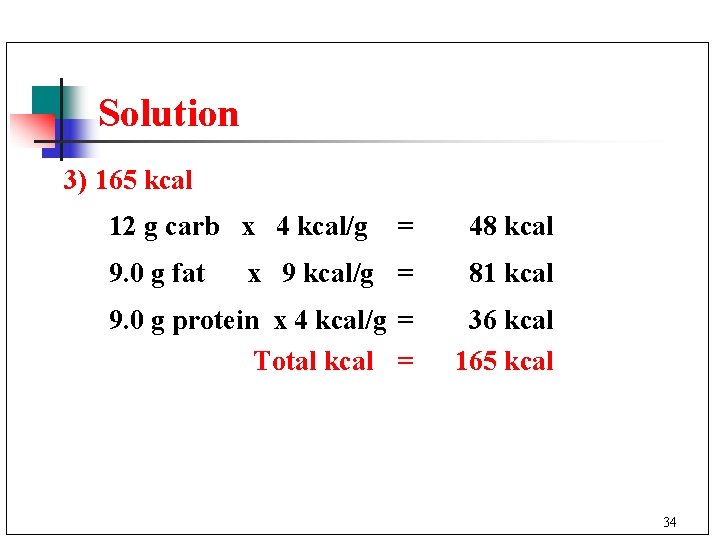
Learning Check A cup of whole milk contains 12 g of carbohydrates, 9. 0 g of fat, and 9. 0 g of protein. How many kcal (Cal) does a cup of milk contain? 1) 48 kcal 2) 81 kcal 3) 165 kcal 33

Solution 3) 165 kcal 12 g carb x 4 kcal/g = 48 kcal x 9 kcal/g = 81 kcal 9. 0 g protein x 4 kcal/g = Total kcal = 36 kcal 165 kcal 9. 0 g fat 34

States of Matter 35

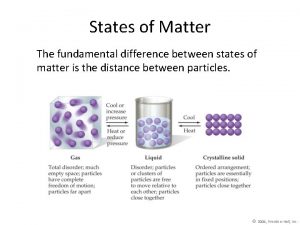
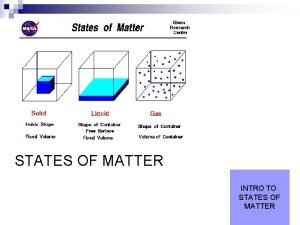
Solids have § A definite shape. § A definite volume. § § Particles that are close together in a fixed arrangement. Particles that move very slowly. 36

Liquids have § An indefinite shape, but a definite volume. § The same shape as their container. § Particles that are close together, but mobile. § Particles that move slowly. 37

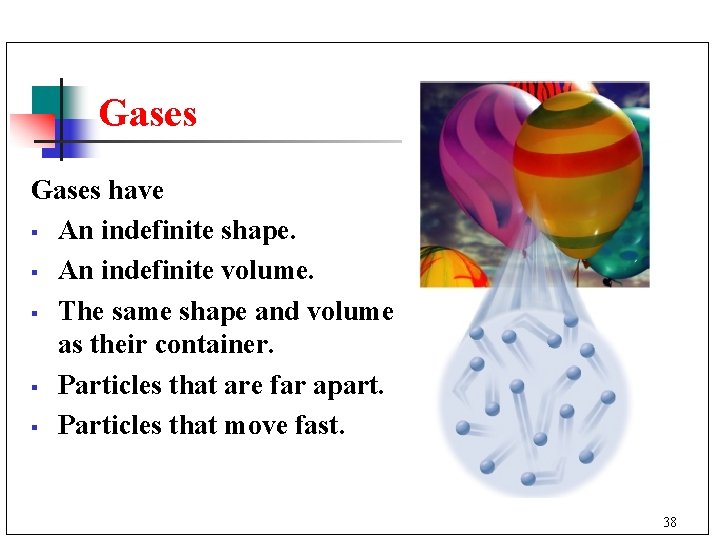
Gases have § An indefinite shape. § An indefinite volume. § The same shape and volume as their container. § Particles that are far apart. § Particles that move fast. 38

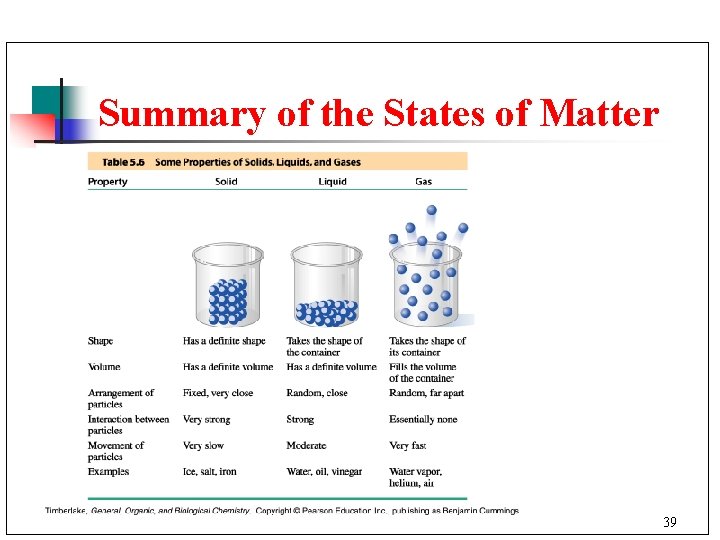
Summary of the States of Matter 39

Learning Check Identify each as: 1) solid 2) liquid or 3) gas. ___ A. It has a definite volume, but takes the shape of the container. __ B. Its particles are moving rapidly. __C. It fills the volume of a container. __D. It has particles in a fixed arrangement. __E. It has particles close together that are mobile. 40

Solution Identify each as: 1) solid 2) liquid or 3) gas. 2 A. It has a definite volume, but takes the shape of the container. 3 B. Its particles are moving rapidly. 3 C. It fills the volume of a container. 1 D. It has particles in a fixed arrangement. 2 E. It has particles close together that are mobile. 41

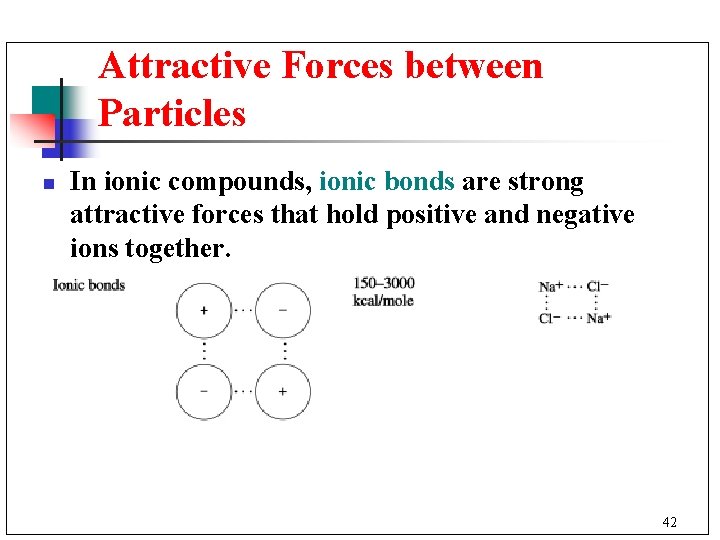
Attractive Forces between Particles n In ionic compounds, ionic bonds are strong attractive forces that hold positive and negative ions together. 42

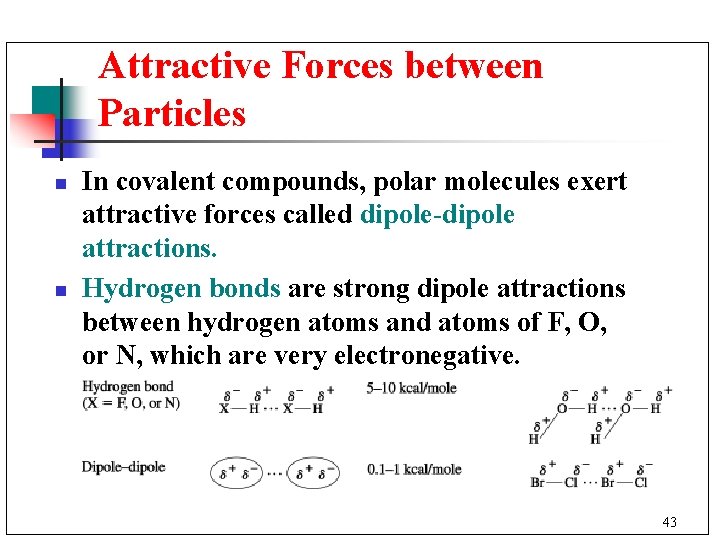
Attractive Forces between Particles n n In covalent compounds, polar molecules exert attractive forces called dipole-dipole attractions. Hydrogen bonds are strong dipole attractions between hydrogen atoms and atoms of F, O, or N, which are very electronegative. 43

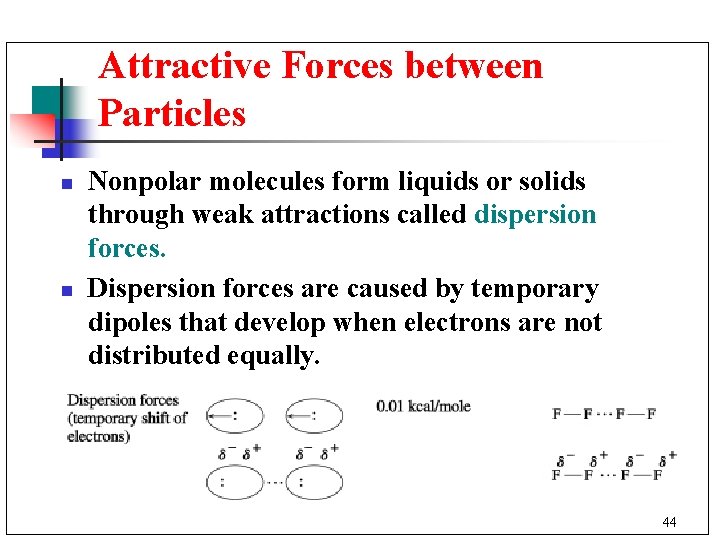
Attractive Forces between Particles n n Nonpolar molecules form liquids or solids through weak attractions called dispersion forces. Dispersion forces are caused by temporary dipoles that develop when electrons are not distributed equally. 44

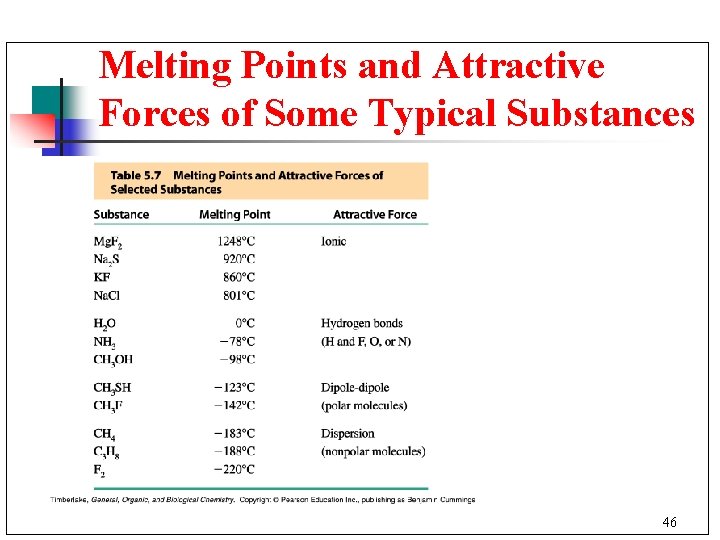
Melting Points and Attractive Forces n n n Ionic compounds require large amounts of energy to break apart ionic bonds. Thus, they have high melting points. Hydrogen bonds are the strongest type of dipole-dipole attractions. They require more energy to break than other dipole attractions. Dispersion forces are weak interactions and very little energy is needed to change state. 45

Melting Points and Attractive Forces of Some Typical Substances 46

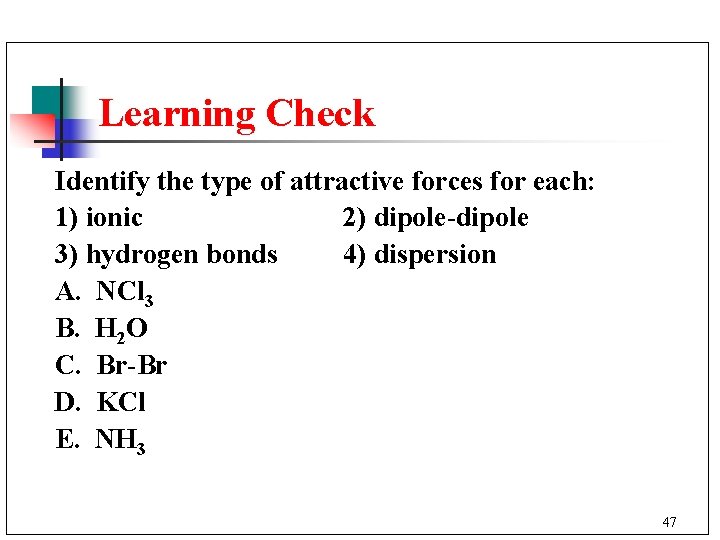
Learning Check Identify the type of attractive forces for each: 1) ionic 2) dipole-dipole 3) hydrogen bonds 4) dispersion A. NCl 3 B. H 2 O C. Br-Br D. KCl E. NH 3 47

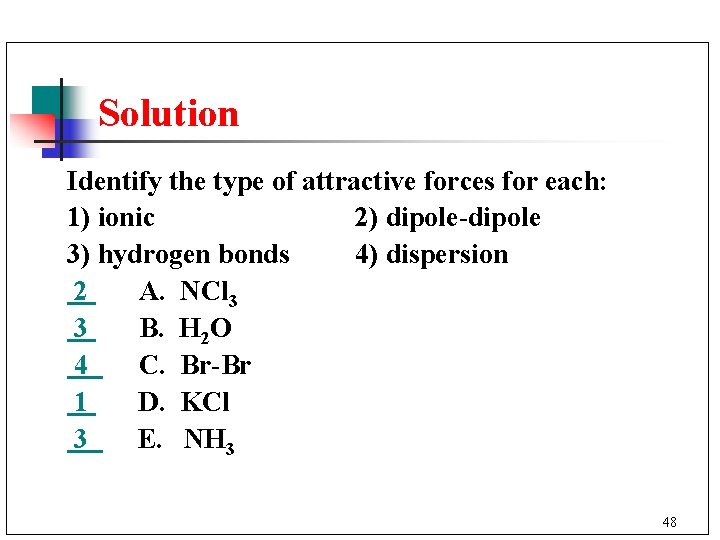
Solution Identify the type of attractive forces for each: 1) ionic 2) dipole-dipole 3) hydrogen bonds 4) dispersion 2 A. NCl 3 3 B. H 2 O 4 C. Br-Br 1 D. KCl 3 E. NH 3 48

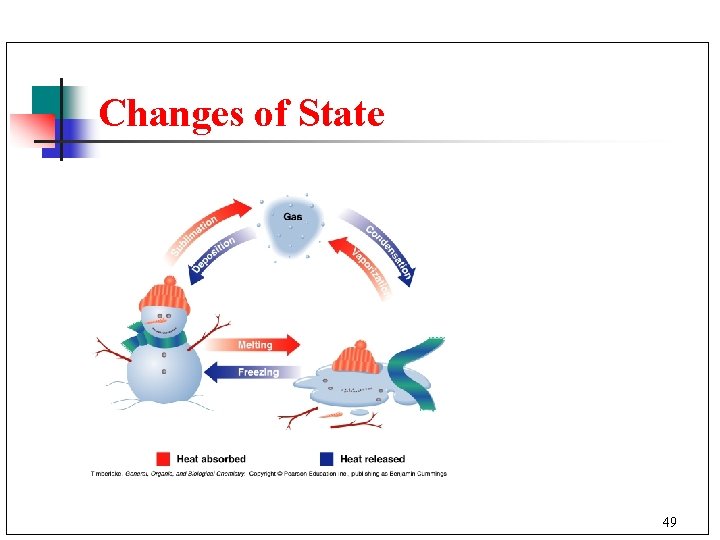
Changes of State 49

Melting and Freezing n n n A substance is melting while it changes from a solid to a liquid. A substance is freezing while it changes from a liquid to a solid. The freezing (melting) point of water is 0°C. 50

Calculations Using Heat of Fusion n The heat of fusion is the amount of heat released when 1 gram of liquid freezes at its freezing point. The heat of fusion is the amount of heat needed to melt 1 gram of a solid at its melting point. For water the heat of fusion (at 0°C) is 80. cal 1 g water 51

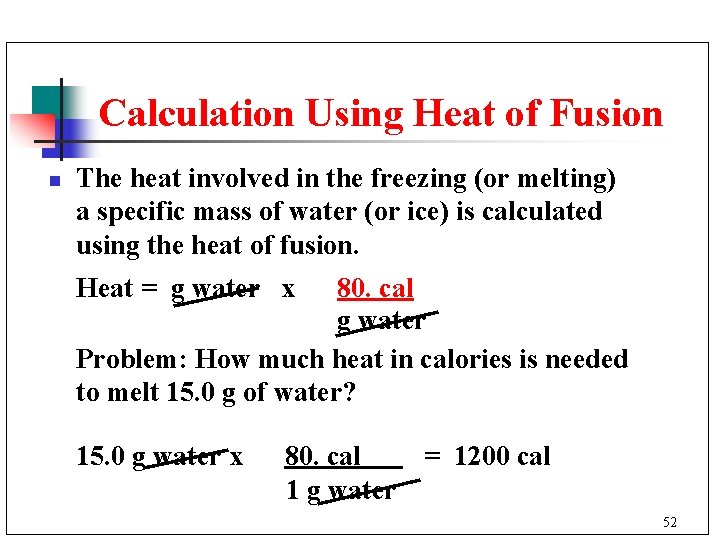
Calculation Using Heat of Fusion n The heat involved in the freezing (or melting) a specific mass of water (or ice) is calculated using the heat of fusion. Heat = g water x 80. cal g water Problem: How much heat in calories is needed to melt 15. 0 g of water? 15. 0 g water x 80. cal = 1200 cal 1 g water 52

Learning Check A. How many calories are needed to melt 5. 0 g of ice of 0°C? 1) 80. cal 2) 400 cal 3) 0 cal B. How many calories are released when 25 g of water at 0°C freezes? 1) 80. cal 2) 0 cal 3) 2000 cal 53

Solution A. How many calories are needed to melt 5. 0 g of ice of 0°C? 2) 400 cal 5. 0 g x 80. cal 1 g B. How many calories are released when 25 g of water at 0°C freezes? 3) 2000 cal 25 g x 80. cal 1 g 54

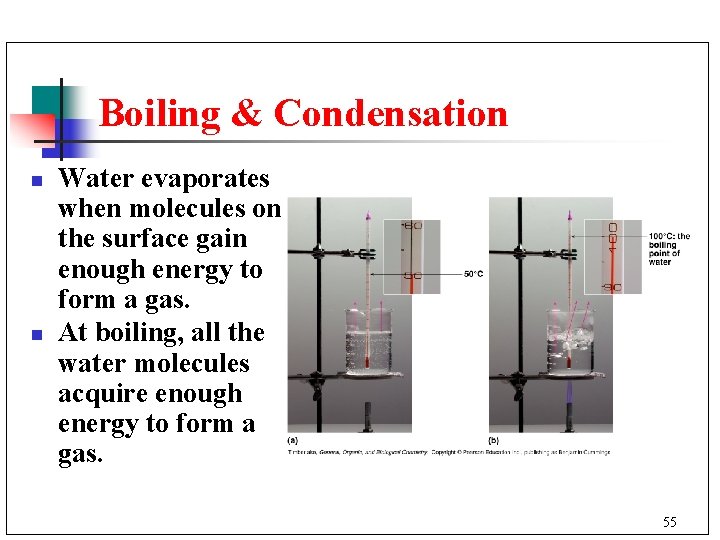
Boiling & Condensation n n Water evaporates when molecules on the surface gain enough energy to form a gas. At boiling, all the water molecules acquire enough energy to form a gas. 55

Heat of Vaporization The heat of vaporization n Is the amount of heat needed to change 1 g of liquid to gas at the boiling point. n Is the amount of heat released when 1 g of a gas changes to liquid at the boiling point. Boiling (Condensing) Point of Water = 100°C Heat of Vaporization (water) = 540 cal 1 g water 56

Learning Check How many kilocalories (kcal) are released when 50. 0 g of steam in a volcano condenses at 100°C? 1) 27 kcal 2) 540 kcal 3) 27 000 kcal 57

Solution How many kilocalories (kcal) are released when 50. 0 g of steam in a volcano condenses at 100°C? 1) 27 kcal 50. 0 g steam x 540 cal x 1 kcal = 27 kcal 1 g steam 1000 cal 58

Heating and Cooling Curves 59

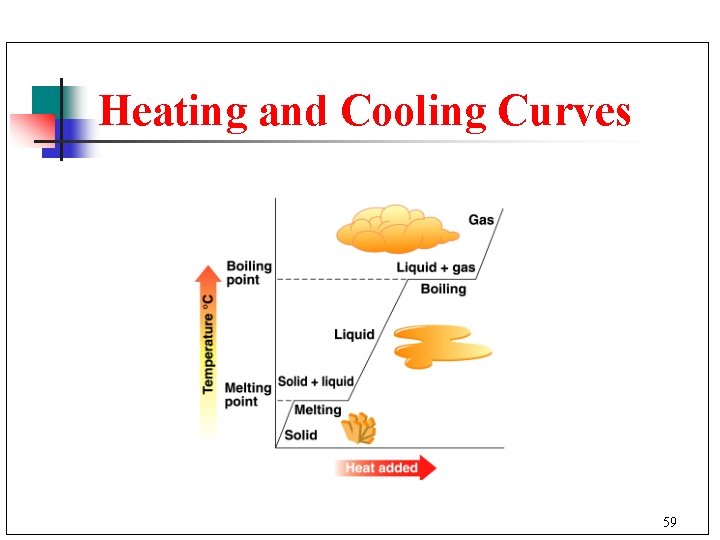
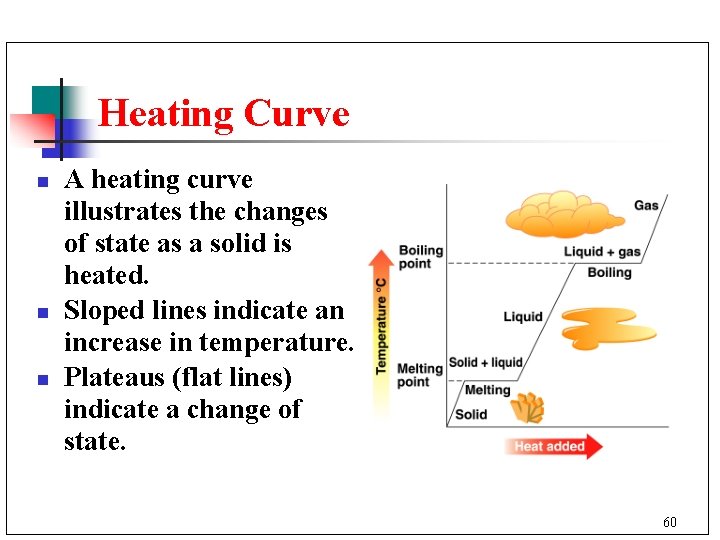
Heating Curve n n n A heating curve illustrates the changes of state as a solid is heated. Sloped lines indicate an increase in temperature. Plateaus (flat lines) indicate a change of state. 60

Learning Check A. A flat line on a heating curve represents 1) a temperature change 2) a constant temperature 3) a change of state B. A sloped line on a heating curve represents 1) a temperature change 2) a constant temperature 3) a change of state 61

Solution A. A flat line on a heating curve represents 2) a constant temperature 3) a change of state B. A sloped line on a heating curve represents 1) a temperature change 62

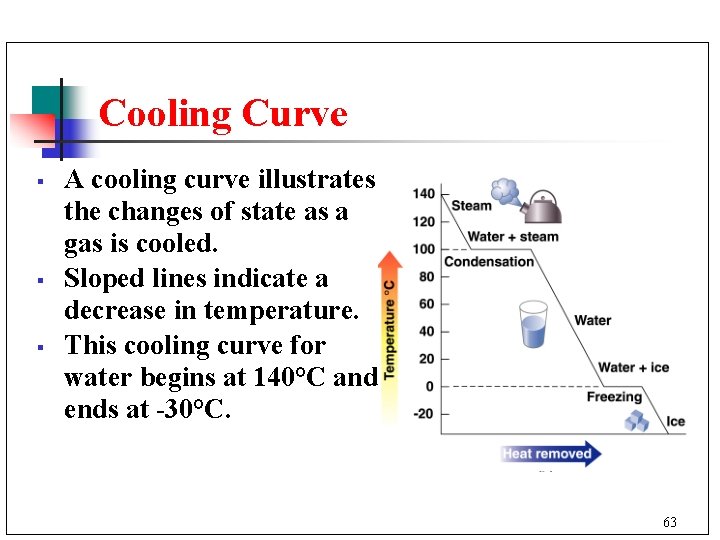
Cooling Curve § § § A cooling curve illustrates the changes of state as a gas is cooled. Sloped lines indicate a decrease in temperature. This cooling curve for water begins at 140°C and ends at -30°C. 63

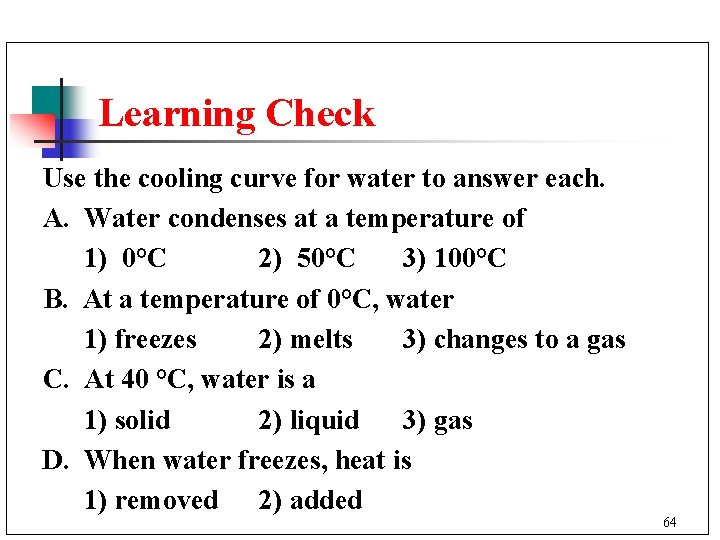
Learning Check Use the cooling curve for water to answer each. A. Water condenses at a temperature of 1) 0°C 2) 50°C 3) 100°C B. At a temperature of 0°C, water 1) freezes 2) melts 3) changes to a gas C. At 40 °C, water is a 1) solid 2) liquid 3) gas D. When water freezes, heat is 1) removed 2) added 64

Solution Use the cooling curve for water to answer each. A. Water condenses at a temperature of 3) 100°C B. At a temperature of 0°C, water 1) freezes C. At 40 °C, water is a 2) liquid D. When water freezes, heat is 1) removed 65

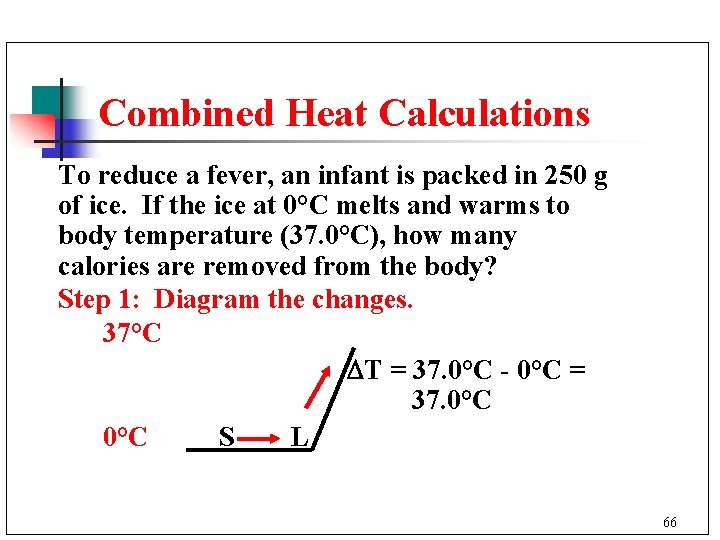
Combined Heat Calculations To reduce a fever, an infant is packed in 250 g of ice. If the ice at 0°C melts and warms to body temperature (37. 0°C), how many calories are removed from the body? Step 1: Diagram the changes. 37°C T = 37. 0°C - 0°C = 37. 0°C S L 66

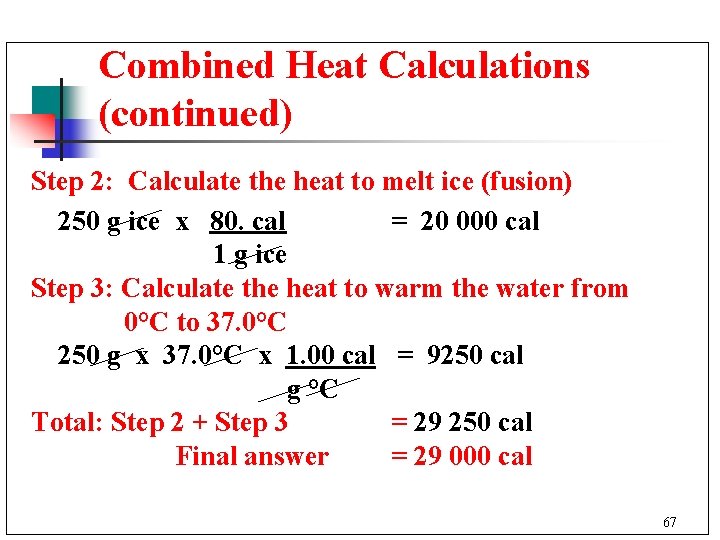
Combined Heat Calculations (continued) Step 2: Calculate the heat to melt ice (fusion) 250 g ice x 80. cal = 20 000 cal 1 g ice Step 3: Calculate the heat to warm the water from 0°C to 37. 0°C 250 g x 37. 0°C x 1. 00 cal = 9250 cal g °C Total: Step 2 + Step 3 = 29 250 cal Final answer = 29 000 cal 67

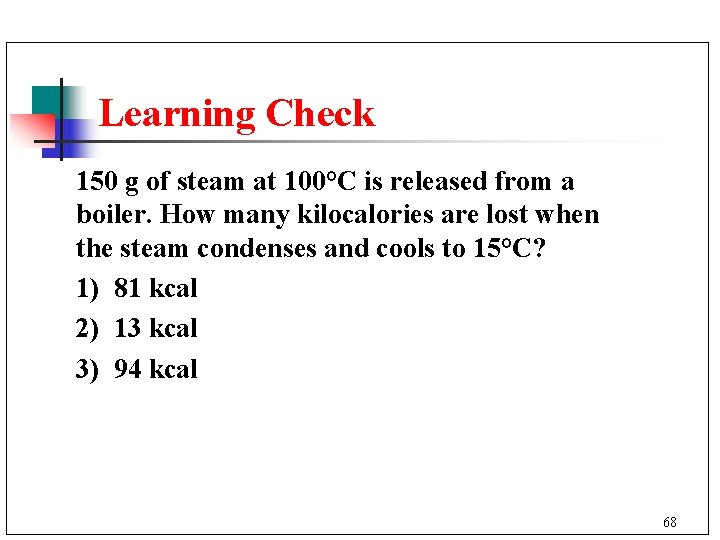
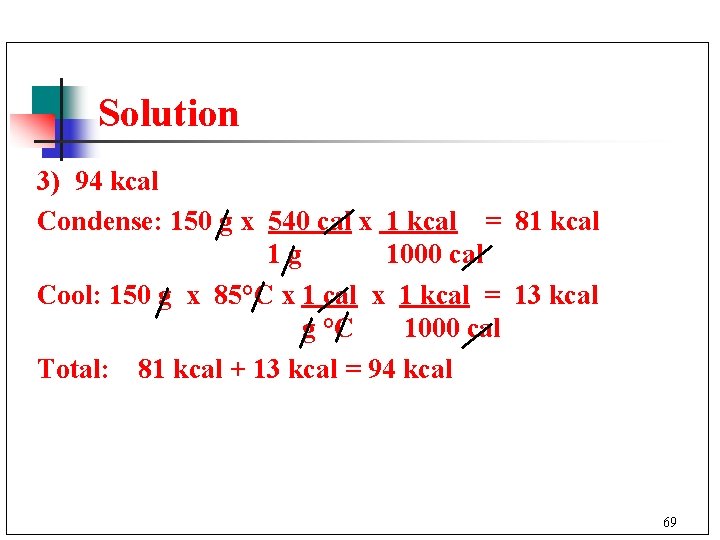
Learning Check 150 g of steam at 100°C is released from a boiler. How many kilocalories are lost when the steam condenses and cools to 15°C? 1) 81 kcal 2) 13 kcal 3) 94 kcal 68

Solution 3) 94 kcal Condense: 150 g x 540 cal x 1 kcal = 81 kcal 1 g 1000 cal Cool: 150 g x 85°C x 1 cal x 1 kcal = 13 kcal g °C 1000 cal Total: 81 kcal + 13 kcal = 94 kcal 69
 Thermal energy states of matter
Thermal energy states of matter Heat thermal energy and temperature
Heat thermal energy and temperature Uses of heat
Uses of heat Flow of energy vs flow of matter
Flow of energy vs flow of matter Plasma particles arrangement
Plasma particles arrangement Gray matter vs white matter
Gray matter vs white matter What makes up the diencephalon
What makes up the diencephalon Gray matter and white matter
Gray matter and white matter Rhinencephalon
Rhinencephalon Chapter 11 - states of matter: liquids and solids
Chapter 11 - states of matter: liquids and solids Whats the study of matter and energy
Whats the study of matter and energy Four phases of matter
Four phases of matter Four states of matter
Four states of matter States of matter
States of matter Changing state
Changing state Phet states of matter basics
Phet states of matter basics 5 states of matter
5 states of matter State of matter venn diagram
State of matter venn diagram The kinetic theory of matter states that
The kinetic theory of matter states that 11 free states
11 free states Southern states vs northern states
Southern states vs northern states Section 1 composition of matter
Section 1 composition of matter Chapter 12 states of matter study guide
Chapter 12 states of matter study guide Chapter 10 review states of matter section 4
Chapter 10 review states of matter section 4 States of matter basics
States of matter basics States of matter foldable
States of matter foldable The fundamental difference between states of matter is the
The fundamental difference between states of matter is the States of matter graph
States of matter graph Section 1 composition of matter
Section 1 composition of matter Phase change concept map solid liquid gas
Phase change concept map solid liquid gas Phases of matter concept map
Phases of matter concept map Whats states of matter
Whats states of matter States of matter
States of matter Properties of matter jeopardy
Properties of matter jeopardy Chapter 2 section 1 classifying matter answer key
Chapter 2 section 1 classifying matter answer key Section 1 composition of matter
Section 1 composition of matter Particle theory of matter examples
Particle theory of matter examples Intro to matter
Intro to matter 4 phases of matter
4 phases of matter States of matter solid liquid gas
States of matter solid liquid gas Interconversion of states of matter
Interconversion of states of matter States of matter flowchart
States of matter flowchart Five states of matter
Five states of matter States of matter objectives
States of matter objectives States of matter
States of matter Solid to gas
Solid to gas All states of matter
All states of matter States of matter jeopardy
States of matter jeopardy 5 states of matter
5 states of matter Jeopardy states of matter
Jeopardy states of matter Big states vs small states guard against tyranny
Big states vs small states guard against tyranny Phosphorus cycle
Phosphorus cycle Section 16.1 thermal energy and matter
Section 16.1 thermal energy and matter Section 1 matter and thermal energy
Section 1 matter and thermal energy Science matter and energy
Science matter and energy Matter energy and measurement
Matter energy and measurement Dark matter and dark energy ppt
Dark matter and dark energy ppt Unit 2 matter and energy
Unit 2 matter and energy Trophic level in an ecosystem represents
Trophic level in an ecosystem represents Lesson outline lesson 1 solids liquids and gases answer key
Lesson outline lesson 1 solids liquids and gases answer key Examples of mechanical wave
Examples of mechanical wave Kesler science properties of waves answer key
Kesler science properties of waves answer key Label the trophic pyramid
Label the trophic pyramid Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis What waves do not require a medium
What waves do not require a medium Wave transfer matter
Wave transfer matter Rhythmic movement that carries energy through matter
Rhythmic movement that carries energy through matter Oikos meaning
Oikos meaning The law of conservation of energy states that
The law of conservation of energy states that