States of Matter There are Four States of









- Slides: 13

States of Matter

There are Four States of Matter • Solid • Liquid • Gas • Plasma

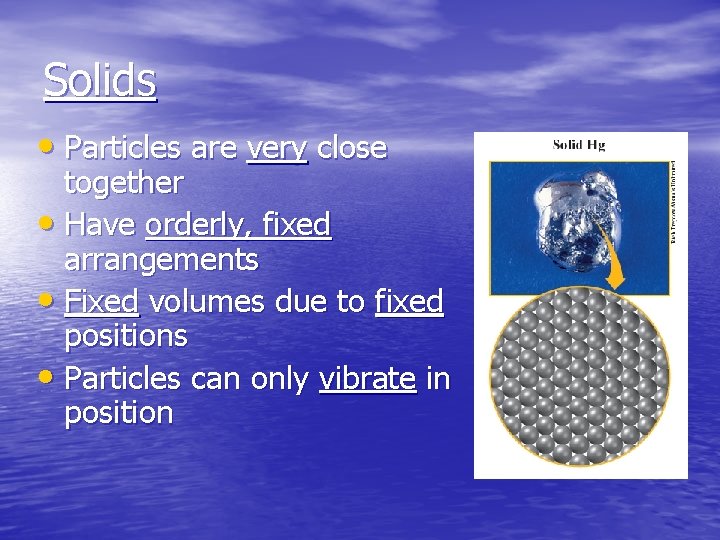
Solids • Particles are very close together • Have orderly, fixed arrangements • Fixed volumes due to fixed positions • Particles can only vibrate in position

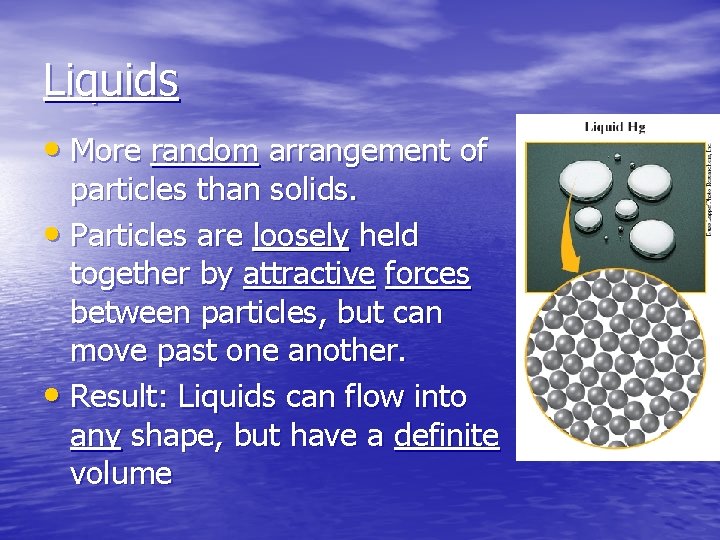
Liquids • More random arrangement of particles than solids. • Particles are loosely held together by attractive forces between particles, but can move past one another. • Result: Liquids can flow into any shape, but have a definite volume

Attractive forces between liquid particles may result in: • Cohesion – Attraction for each other • Adhesion – Attraction to other materials • Capillary Action – Ability to “climb” due to cohesion and adhesion • Surface Tension – Force that act on the surface of a liquid and that tends to minimize the area of the surface

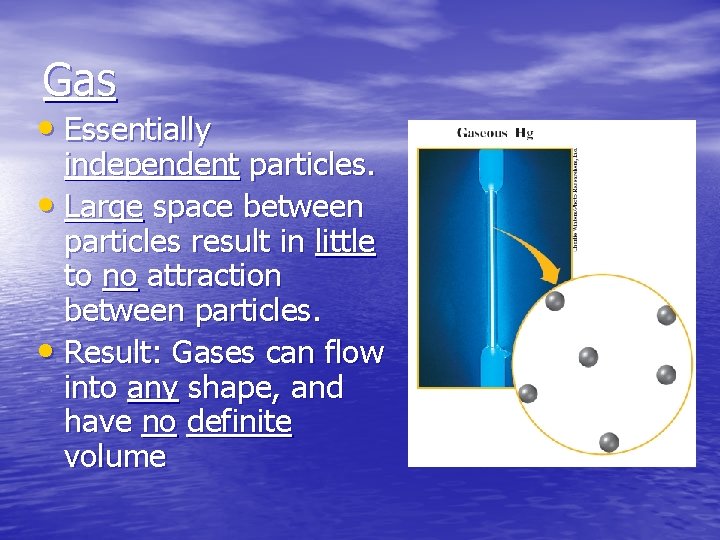
Gas • Essentially independent particles. • Large space between particles result in little to no attraction between particles. • Result: Gases can flow into any shape, and have no definite volume

Plasma • Supercritical fluid • Occurs at very high temperatures and very high pressures • Has properties of both the liquid phase and the gas phase

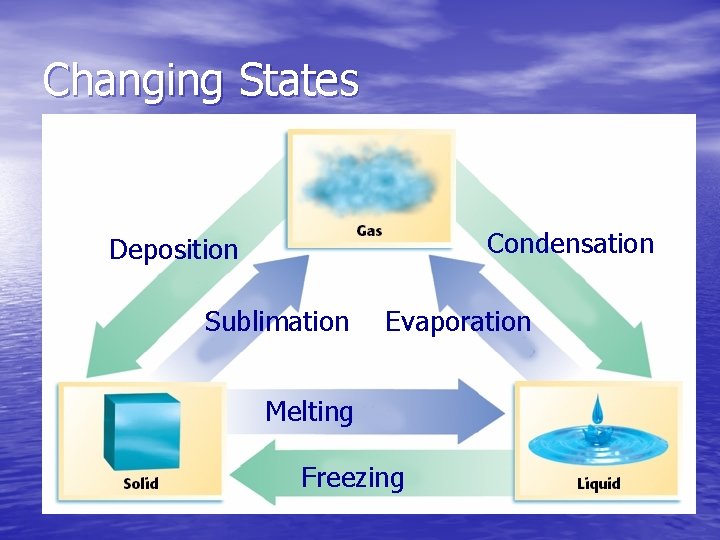
Changing States Condensation Deposition Sublimation Evaporation Melting Freezing

Chemical Changes verses Physical Changes

Physical Changes • A change of matter from one form to another • • without a change in chemical properties A A Does the chemical nature of the substance change? – No • Examples: phase changes, making solutions

Chemical Changes • A change that occurs when one or more • • substances change into entirely new substances with different properties A + B C (reactants go to products) Does the chemical nature of the substance change? – Yes – Example: Electrolysis of hydrogen and oxygen gases to make water

Release or Absorption of Energy Formation of a Precipitate a gas (solid) An Unexpected Color Change

Chemical or Physical • Frying an egg - Chemical • Boiling Water - Physical • Sanding a wooden plank - Physical • Digesting food - Chemical • Popping a balloon - Physical
 Antigentest åre
Antigentest åre Four phases of matter
Four phases of matter Four states of matter
Four states of matter Plasma particles arrangement
Plasma particles arrangement 4 phases of matter
4 phases of matter Whats the four states of matter
Whats the four states of matter Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Grey matter of nervous system
Grey matter of nervous system Composition of matter section 1
Composition of matter section 1 Chapter 2 section 1 classifying matter answer key
Chapter 2 section 1 classifying matter answer key Gray matter and white matter
Gray matter and white matter Section 1 composition of matter chapter 15 answer key
Section 1 composition of matter chapter 15 answer key Gray matter and white matter
Gray matter and white matter Ncl. caudatus
Ncl. caudatus