States of Matter 3 States of Matter n





- Slides: 18

States of Matter

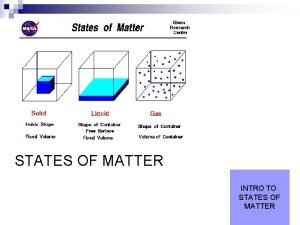
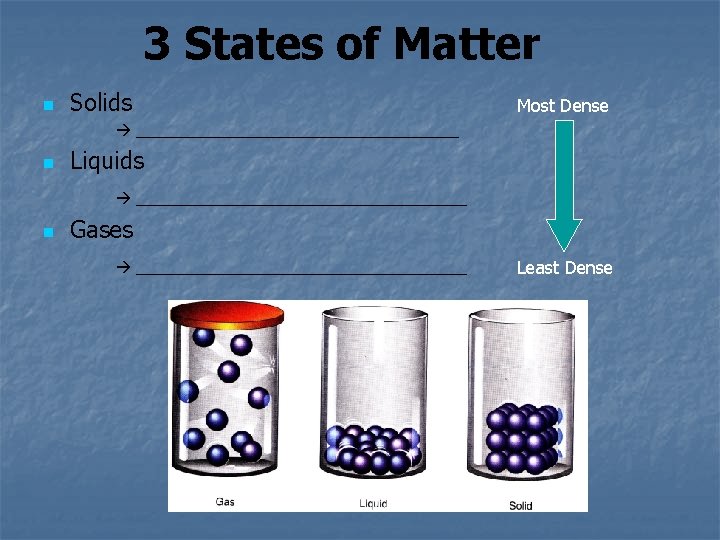
3 States of Matter n Solids Most Dense ___________________ n Liquids ___________________ n Gases ___________________ Least Dense

The 4 th State of Matter n What happens if you raise the temperature to super-high levels…between 1000°C and 1, 000, 000°C? PLASMA!! § § § A plasma is an ionized gas. A plasma is a very good conductor of electricity and is affected by magnetic fields. Plasma, like gases have an indefinite shape and an indefinite volume.

Nature of Gases n n Minimal attractive forces between particles Particles have lots of energy and move freely Kinetic Molecular Theory: = a model or theory used to describe the behavior of gases 1. ) The particles in a gas are considered to be __________________________________________ 2. ) The motion of the particles in a gas is ___________________________________________ 3. ) All collisions between particles in a gas are perfectly elastic _________________________________________________________

Gas Pressure n Gas pressure = _______________________________________ **atmospheric pressure = ___________________________ measured with a barometer Units: kilopascal (k. Pa) mm. Hg atm torr 1 atm = 101. 3 k. Pa = 760 mm. Hg = 760 torr

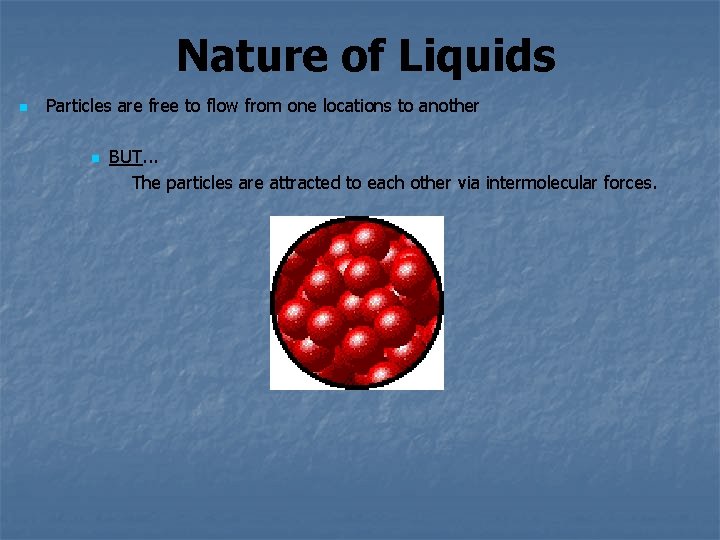
Nature of Liquids n Particles are free to flow from one locations to another n BUT. . . The particles are attracted to each other via intermolecular forces.

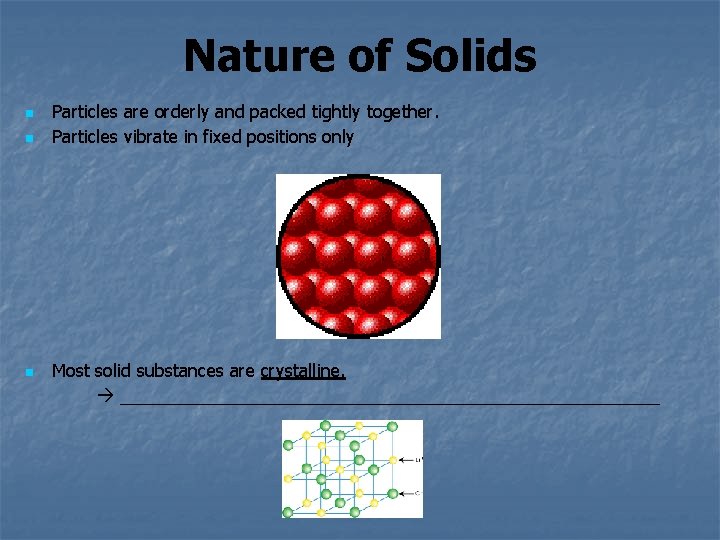
Nature of Solids n n n Particles are orderly and packed tightly together. Particles vibrate in fixed positions only Most solid substances are crystalline. ____________________________

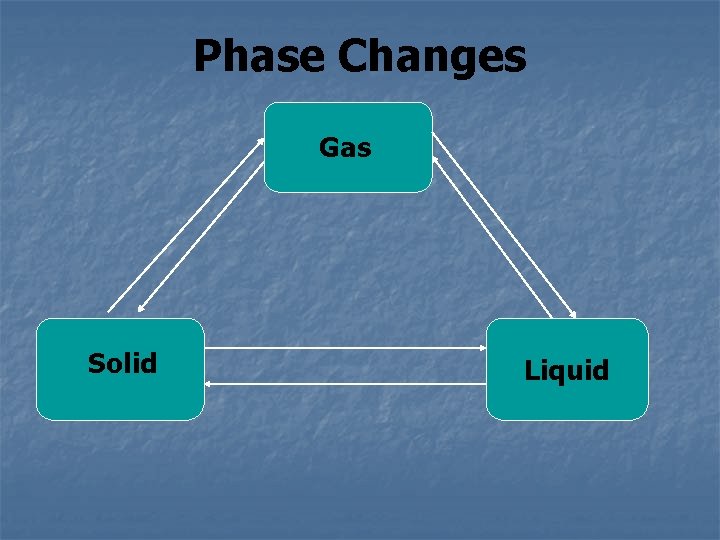
Phase Changes Gas Solid Liquid

Temperature = a measure of the average _________ of particles = ___________ **At a given temperature, the particles of all substances, regardless of physical state, have the same average kinetic energy. ** Kelvin Scale =________________________ boiling point of water = ____ K freezing pt of water = ____ K Reference Tables: absolute zero = ____ K - Table A (STP) - Table T (degrees Celcius to Kelvin

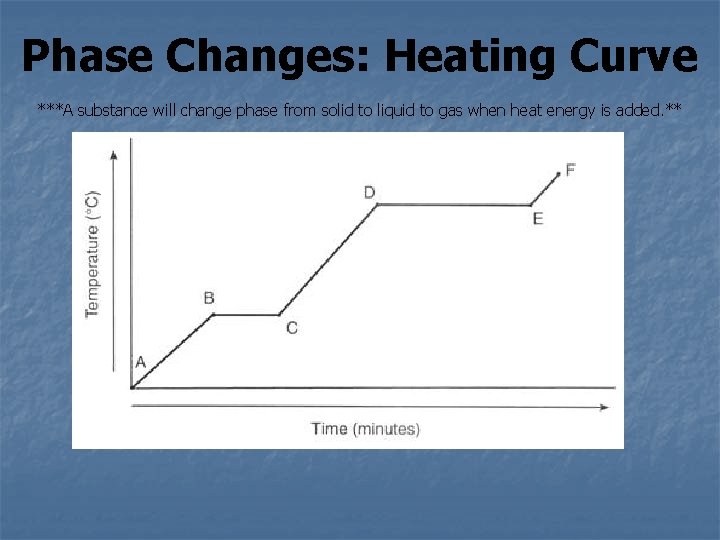
Phase Changes: Heating Curve ***A substance will change phase from solid to liquid to gas when heat energy is added. **

Phase Changes: Cooling Curve ***A substance will change phase from gas to liquid to solid when heat energy is lost. **

Heating/Cooling Curve Combined


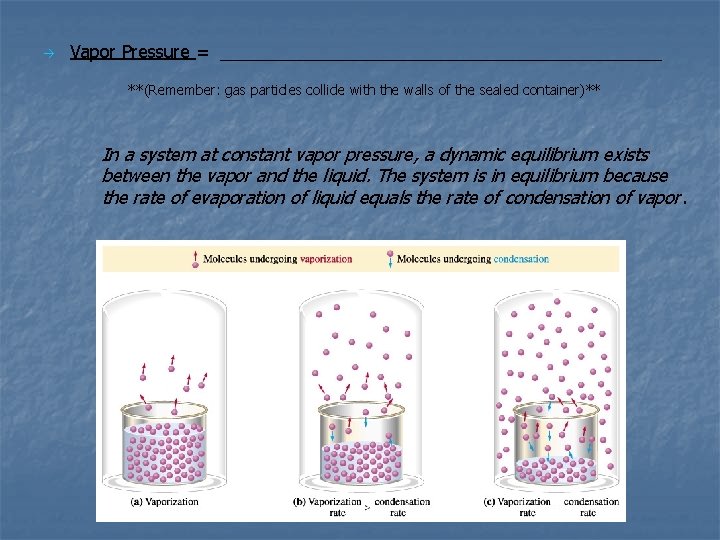
Vapor Pressure = _______________________ **(Remember: gas particles collide with the walls of the sealed container)** In a system at constant vapor pressure, a dynamic equilibrium exists between the vapor and the liquid. The system is in equilibrium because the rate of evaporation of liquid equals the rate of condensation of vapor.

Vapor Pressure & Temperature: How will temperature affect vapor pressure? _______________________ Why? _______________________________________________ **The higher the vapor pressure of a substance, the more volatile it is. volatility = _______________

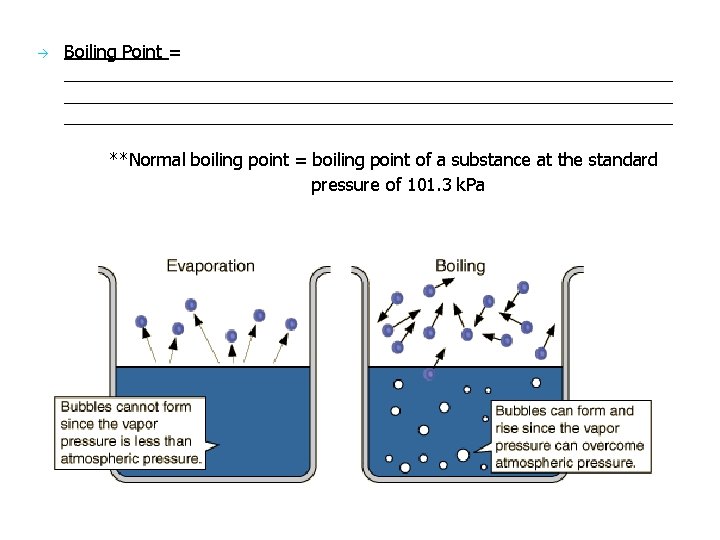
Boiling Point = ______________________________________________________________ **Normal boiling point = boiling point of a substance at the standard pressure of 101. 3 k. Pa

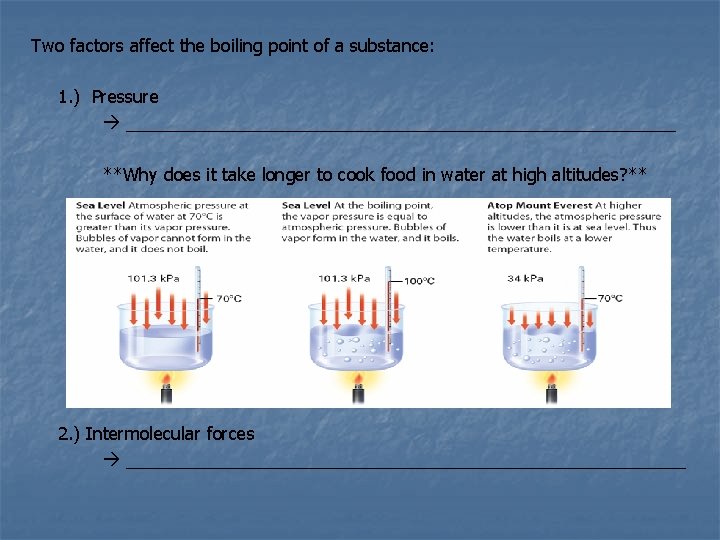
Two factors affect the boiling point of a substance: 1. ) Pressure ____________________________ **Why does it take longer to cook food in water at high altitudes? ** 2. ) Intermolecular forces _____________________________

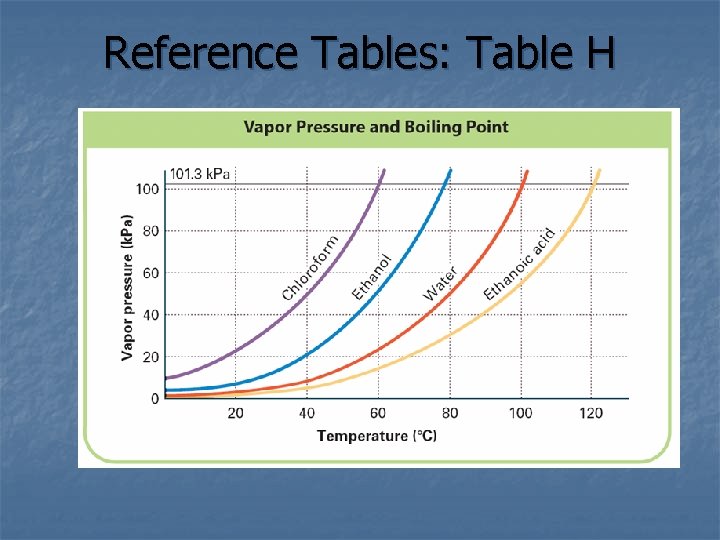
Reference Tables: Table H
 Energy naturally flows from warmer matter to cooler matter
Energy naturally flows from warmer matter to cooler matter The change of phase from gas to solid *
The change of phase from gas to solid * Interconversion of states of matter
Interconversion of states of matter States of matter
States of matter Venn diagram of conduction convection and radiation
Venn diagram of conduction convection and radiation Median and lateral apertures
Median and lateral apertures Stayes of matter
Stayes of matter Four phases of matter
Four phases of matter Matter
Matter Section 1 composition of matter
Section 1 composition of matter All matter is in constant motion
All matter is in constant motion Phase change concept map
Phase change concept map Changing state
Changing state Thermal energy in states of matter
Thermal energy in states of matter Jeopardy states of matter
Jeopardy states of matter Matter flow chart
Matter flow chart Properties of matter jeopardy
Properties of matter jeopardy The kinetic theory of matter states that
The kinetic theory of matter states that Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter