States of Matter The Four States of Matter













- Slides: 16

States of Matter The Four States of Matter 1

States of Matter The Four States of Matter Four States u. Solid u. Liquid u. Gas u. Plasma 2

States of Matter The Four States of Matter ØBased upon particle arrangement ØBased upon energy of particles ØBased upon distance between particles ØBased on intermolecular forces between particles. 3

States of Matter Solids §Particles of solids are tightly packed, vibrating about a fixed position. §Solids have a definite shape and a definite volume. §Strong intermolecular force. 4

States of Matter Solids Particle Movement Examples 5

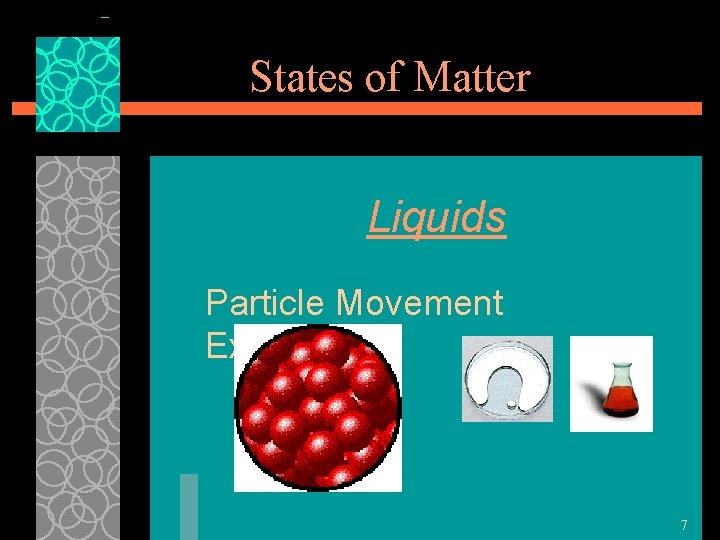
States of Matter Liquids §Particles of liquids are tightly packed, but are far enough apart to slide over one another. §Liquids have an indefinite shape and a definite volume. §Strong intermolecular force, but weaker than a solid. 6

States of Matter Liquids Particle Movement Examples 7

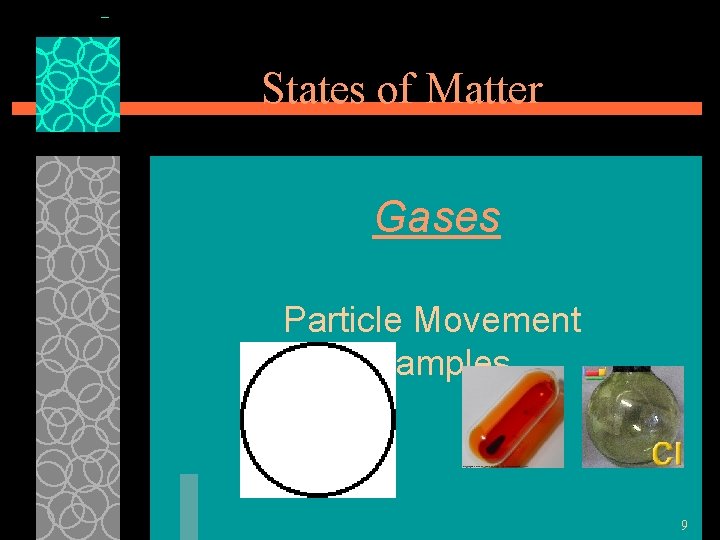
States of Matter Gases §Particles of gases are very far apart and move freely. §Gases have an indefinite shape and an indefinite volume. §Little or no intermolecular force. 8

States of Matter Gases Particle Movement Examples 9

States of Matter Plasma §A plasma is an ionized gas. §A plasma is a very good conductor of electricity and is affected by magnetic fields. §Plasma, like gases have an indefinite shape and an indefinite volume. 10

States of Matter Plasma Particles The negatively charged electrons (yellow) are freely streaming through the positively charged ions (blue). 11

States of Matter Plasma Examples 12

States of Matter Compressibility §Solids cannot be compressed because they are closely packed together. §Liquids cannot be compressed but are more compressible than solids. §Gases and Plasma are easily compressible because there is a great deal of free space between particles. 13

States of Matter The Four States of Matter The Classification and Properties of Matter Depend Upon Microscopic Structure ØParticle arrangement ØParticle energy 14

States of Matter Kinetic Theory According to the kinetic theory of matter, matter is made of atoms and molecules. These atoms and molecules act like tiny particles that are always in motion. The kinetic theory helps to explain the differences between the three common states of matter: solid, liquid, and gas. 15

States of Matter The following are observations of particles in motion: – The higher the temperature of the substance is, the faster the particles move. – At the same temperature, more massive particles move slower than less massive ones. 16