States of Matter I States of Matter A






- Slides: 17

States of Matter

I. States of Matter A. Matter can be found in 4 states: 1. Solid 2. Liquid 3. Gas 4. Plasma

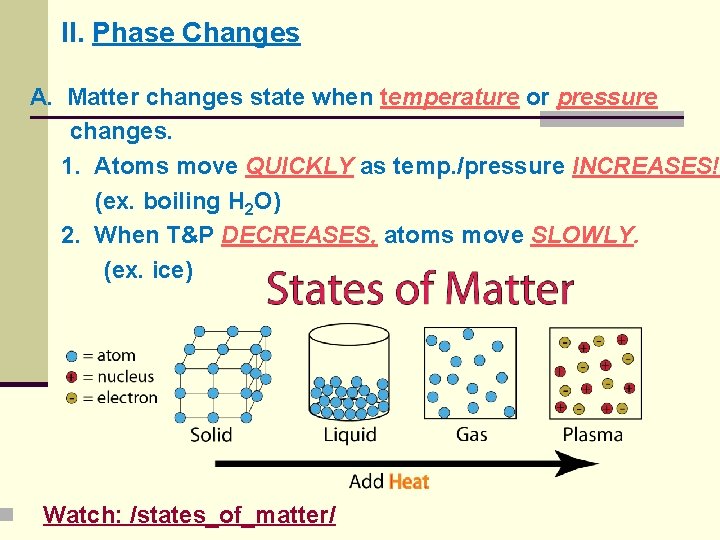
B. STATE OF MATTER DEPENDS ON: 1. particle arrangement 2. temperature (energy) of particles 3. distance between particles

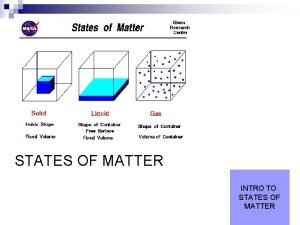
C. SOLIDS 1. Particles are tightly packed in a fixed position 2. Particles vibrate against one another 3. Have a definite shape & a definite volume. Particle Movement: Examples:

D. LIQUIDS 1. Particles slide past one another. 2. Particles take on the shape of their container 3. Have an indefinite (no) shape and a definite volume. Particle Movement: Examples:

E. GASES 1. Particles are very far apart and move freely and quickly. 2. Particles can escape from their containers. 3. Have an indefinite shape and an indefinite volume Particle Movement: Examples:

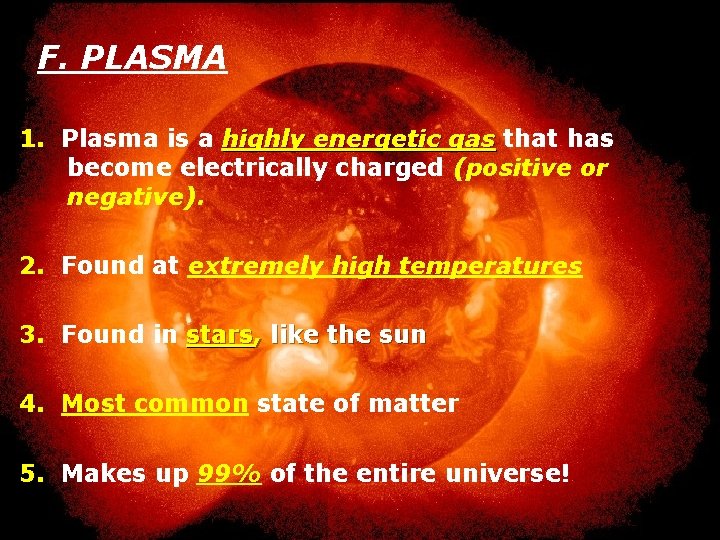
F. PLASMA 1. Plasma is a highly energetic gas that has become electrically charged (positive or negative). 2. Found at extremely high temperatures 3. Found in stars, like the sun 4. Most common state of matter 5. Makes up 99% of the entire universe!

6. Plasma also has an indefinite shape and volume. 7. Lightning is a natural form of plasma on Earth. The negatively charged electrons (red) are freely moving through the positively charged ions (green).

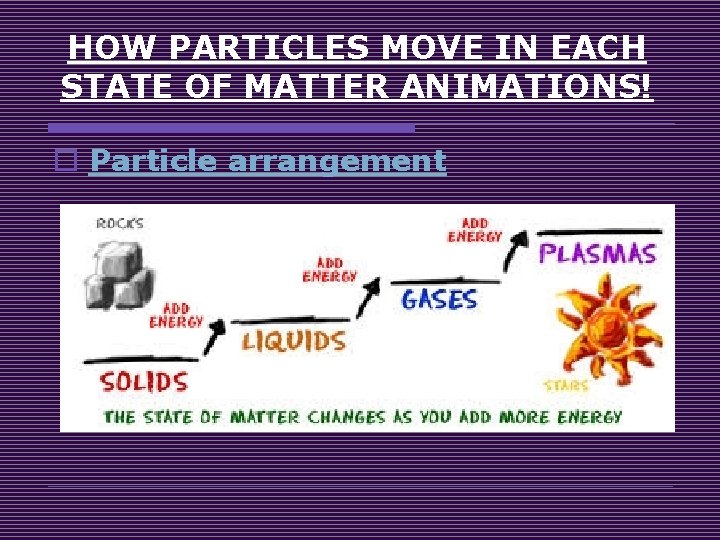
HOW PARTICLES MOVE IN EACH STATE OF MATTER ANIMATIONS! o Particle arrangement

REVIEW: Identify each state of matter. 1. 5. 2. 6. No definite shape or volume 7. Definite shape, definite volume 3. 8. indefinite shape, definite volume 9. particles are tightly packed & vibrate 4. 10. particles move quickly & can escape their container 11. particles can move past one, but take the shape of their container

n II. Phase Changes A. Matter changes state when temperature or pressure changes. 1. Atoms move QUICKLY as temp. /pressure INCREASES! (ex. boiling H 2 O) 2. When T&P DECREASES, atoms move SLOWLY. (ex. ice) Watch: /states_of_matter/

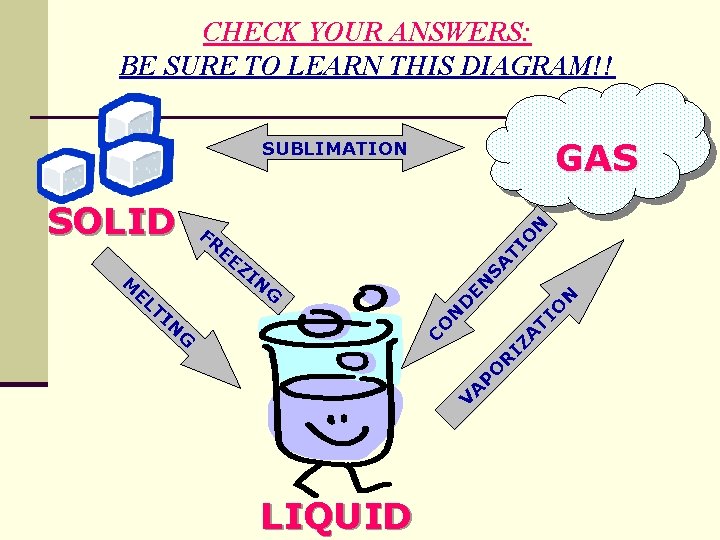
B. Phase Change Processes (Use pgs. 50 -55 to name each process) 1. solid 2. 3. 4. 5. 6. 7. 8. liquid = _______ liquid solid = _______ liquid gas = _______ (occurs at surface) liquid gas = _______ (below surface, bubbles) gas liquid = _______ gas solid = _______ solid gas = _______ An example of sublimation is ______. n. View: 1. /states_of_matter/ 2. changing phases

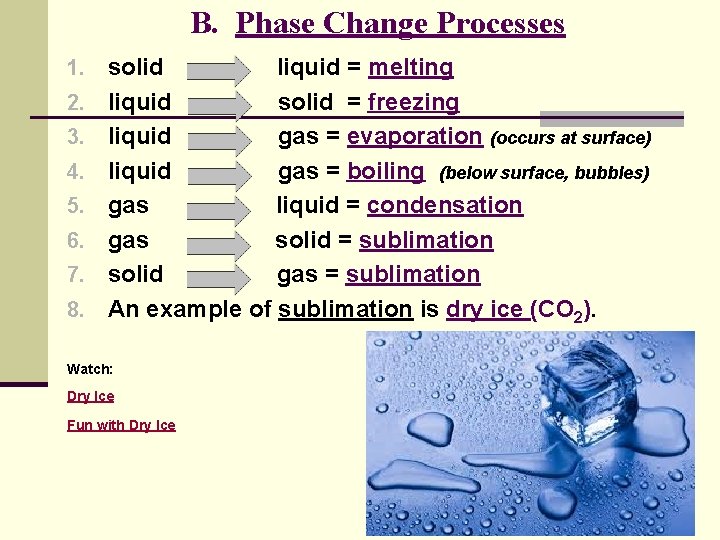
B. Phase Change Processes 1. solid 2. 3. 4. 5. 6. 7. 8. liquid = melting liquid solid = freezing liquid gas = evaporation (occurs at surface) liquid gas = boiling (below surface, bubbles) gas liquid = condensation gas solid = sublimation solid gas = sublimation An example of sublimation is dry ice (CO 2). Watch: Dry Ice Fun with Dry Ice

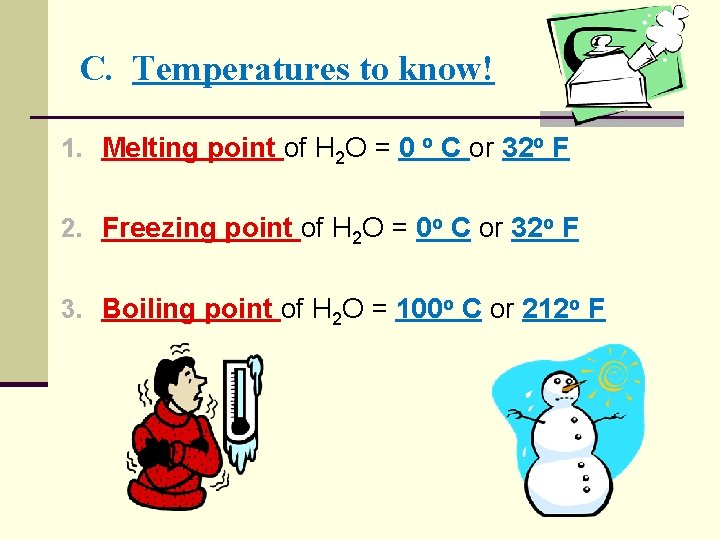
C. Temperatures to know! 1. Melting point of H 2 O = _____o C or _____o F 2. Freezing point of H 2 O = _____o C or _____o F 3. Boiling point of H 2 O = _____o C &or _____o F

C. Temperatures to know! 1. Melting point of H 2 O = 0 o C or 32 o F 2. Freezing point of H 2 O = 0 o C or 32 o F 3. Boiling point of H 2 O = 100 o C or 212 o F

D. Phase Changes Diagram: Label each arrow with the appropriate phase change. GAS SOLID LIQUID

CHECK YOUR ANSWERS: BE SURE TO LEARN THIS DIAGRAM!! GAS EE M SA ZI N N N TI O D CO G V A P O R IZ A TI EN G EL N FR TI O SOLID N SUBLIMATION LIQUID
 Ncl caudatus
Ncl caudatus States of matter diagram
States of matter diagram All matter is in constant motion
All matter is in constant motion Concept map states of matter
Concept map states of matter Four states of matter
Four states of matter Thermal energy vs heat
Thermal energy vs heat Jeopardy states of matter
Jeopardy states of matter Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Flowchart of how matter is classified
Flowchart of how matter is classified Properties of matter jeopardy
Properties of matter jeopardy Changing states of matter
Changing states of matter Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Solid liquid gas plasma
Solid liquid gas plasma States of matter
States of matter Vocab
Vocab The kinetic theory of matter states that
The kinetic theory of matter states that 4 phases of matter
4 phases of matter Concept map of states of matter
Concept map of states of matter