STATES OF MATTER The Four States of Matter


















- Slides: 30


STATES OF MATTER The Four States of Matter Four States Solid Liquid Gas Plasma

STATES OF MATTER ØBased upon particle arrangement ØBased upon energy of particles ØBased upon distance between particles

KINETIC THEORY OF MATTER Matter is made up of particles which are in continual random motion.

STATES OF MATTER SOLIDS • Particles of solids are tightly packed, vibrating about a fixed position. • Solids have a defiite shape and a definite volume. Heat

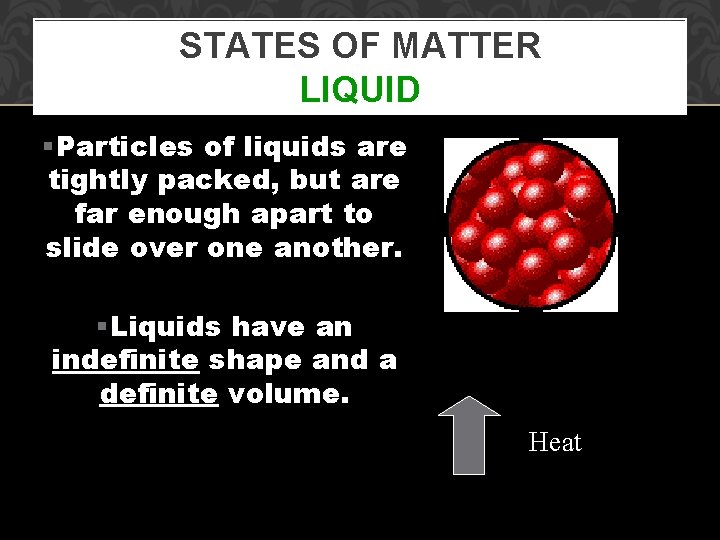
STATES OF MATTER LIQUID §Particles of liquids are tightly packed, but are far enough apart to slide over one another. §Liquids have an indefinite shape and a definite volume. Heat

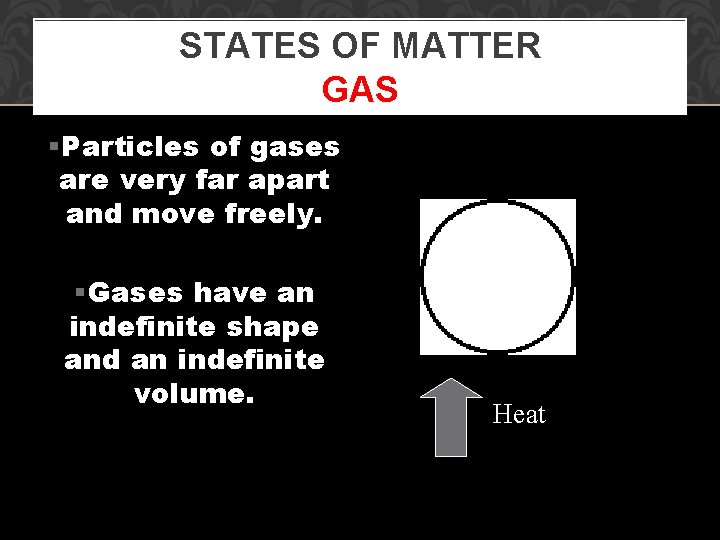
STATES OF MATTER GAS §Particles of gases are very far apart and move freely. §Gases have an indefinite shape and an indefinite volume. Heat

F. PHASE CHANGES – PHYSICAL Evaporation = Liquid -> Gas Condensation = Gas -> Liquid Melting = Solid -> Liquid Freezing = Liquid -> Solid Sublimation = Solid -> Gas

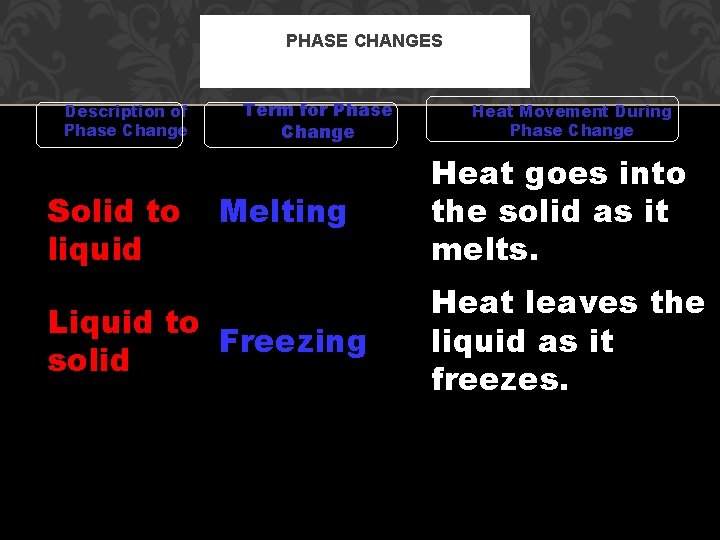
PHASE CHANGES Description of Phase Change Solid to liquid Term for Phase Change Melting Liquid to Freezing solid Heat Movement During Phase Change Heat goes into the solid as it melts. Heat leaves the liquid as it freezes.

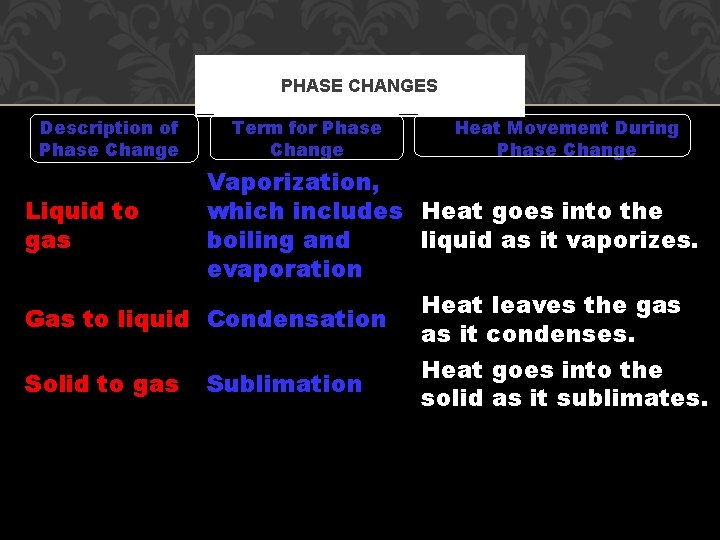
PHASE CHANGES Description of Phase Change Liquid to gas Term for Phase Change Vaporization, which includes Heat goes into the boiling and liquid as it vaporizes. evaporation Gas to liquid Condensation Solid to gas Heat Movement During Phase Change Sublimation Heat leaves the gas as it condenses. Heat goes into the solid as it sublimates.

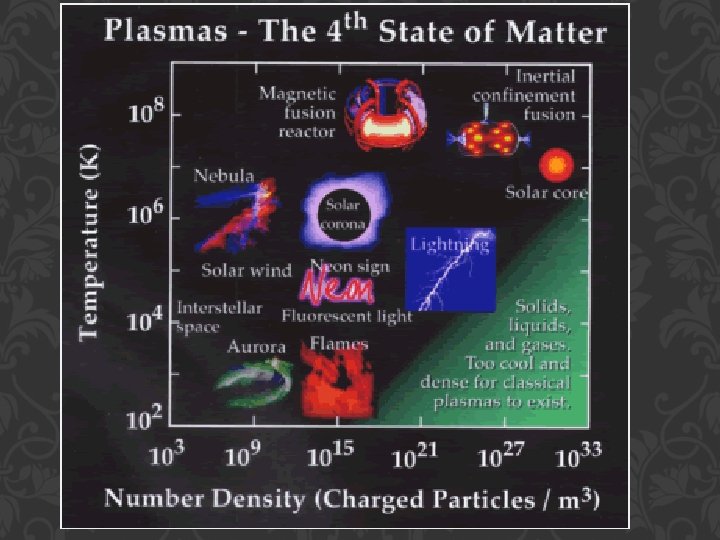
BUT WHAT HAPPENS IF YOU RAISE THE TEMPERATURE TO SUPERHIGH LEVELS… BETWEEN 1000°C AND 1, 000, 000°C ? Will everything just be a gas?

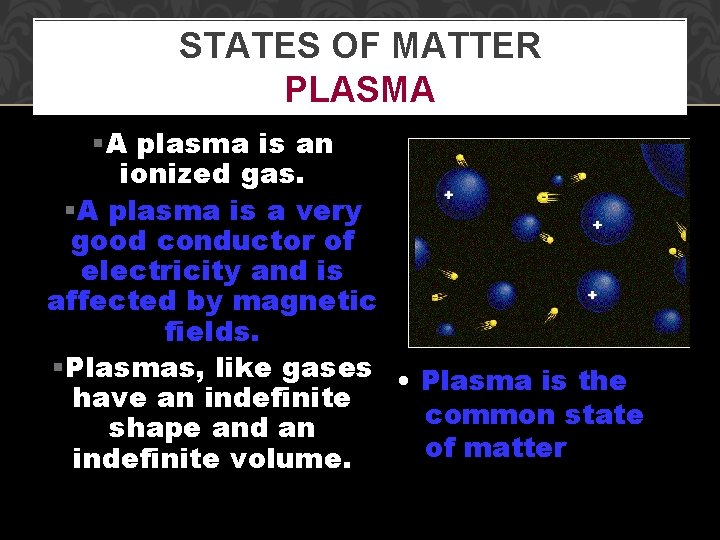
STATES OF MATTER PLASMA §A plasma is an ionized gas. §A plasma is a very good conductor of electricity and is affected by magnetic fields. §Plasmas, like gases • Plasma is the have an indefinite common state shape and an of matter indefinite volume.

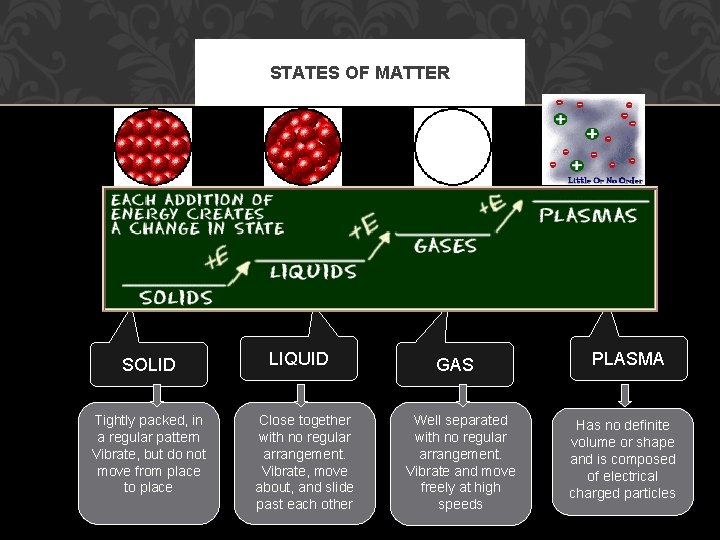
STATES OF MATTER SOLID Tightly packed, in a regular pattern Vibrate, but do not move from place to place LIQUID Close together with no regular arrangement. Vibrate, move about, and slide past each other GAS Well separated with no regular arrangement. Vibrate and move freely at high speeds PLASMA Has no definite volume or shape and is composed of electrical charged particles

SOME PLACES WHERE PLASMAS ARE FOUND… 1. Flames

2. Lightning

3. Aurora (Northern Lights)

The Sun is an example of a star in its plasma state


G. CHEMICAL CHANGES Signs of a Chemical Change change in color or odor formation of a gas formation of a precipitate (solid) change in light or heat

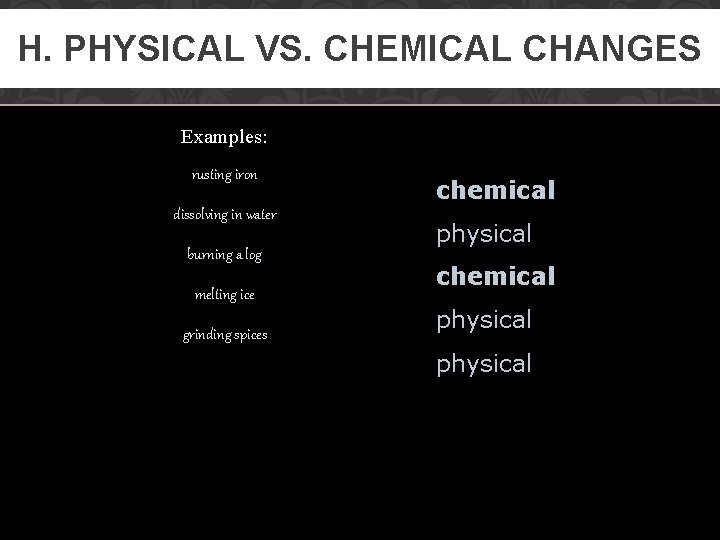
H. PHYSICAL VS. CHEMICAL CHANGES Examples: rusting iron dissolving in water burning a log melting ice grinding spices chemical physical

LEARNING CHECK S 1 Match: (1) solid, (2) liquid, or (3) gas. ____ A. Has a definite volume, but shape of the container. ____ B. Its particles are moving rapidly. ____ C. Fills the volume of a container. ____ D. Particles are in a fixed structure. ____ E. Particles are close together, but mobile. 21

HEAT CALCULATION FOR FUSION Heat = g water x = g water 80. cal g water x 334 J g water How much heat in calories is needed to melt 15. 0 g of water? 15. 0 g water x 80. cal 22 = 1200 cal 1 g water

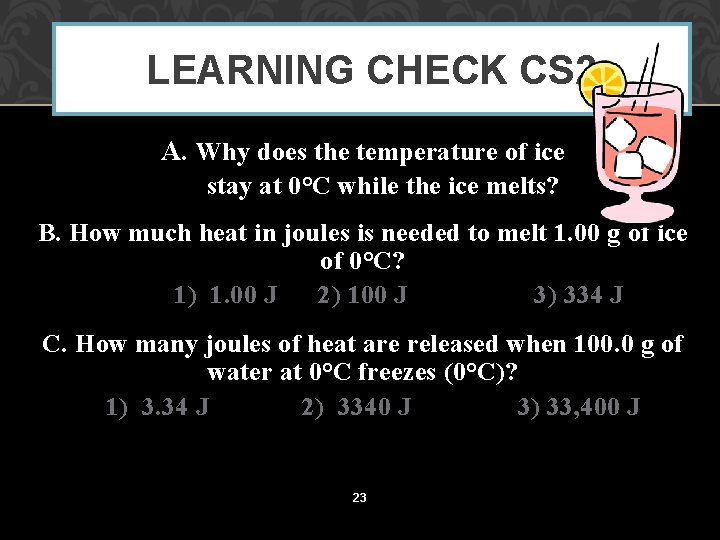
LEARNING CHECK CS 2 A. Why does the temperature of ice stay at 0°C while the ice melts? B. How much heat in joules is needed to melt 1. 00 g of ice of 0°C? 1) 1. 00 J 2) 100 J 3) 334 J C. How many joules of heat are released when 100. 0 g of water at 0°C freezes (0°C)? 1) 3. 34 J 2) 3340 J 3) 33, 400 J 23

SOLUTION CS 2 A. Why does the temperature of ice stay at 0°C while the ice melts? Energy goes into the change of state. B. How much heat in joules is needed to melt 1. 00 g of ice of 0°C? 3) 334 J C. How many joules of heat are released when 100. 0 g of water at 0°C freezes (0°C)? 3) 33, 400 J 24

HEAT OF VAPORIZATION Amount of heat needed to change 1 gram of liquid to gas at its boiling point Boiling (Condensing) Point of Water = 100°C Heat of Vaporization (water) = 2260 J/g 25

LEARNING CHECK CS 4 A. Ice cubes in a warm drink will 1) melt 2) freeze 3) not change B. The liquid drink _____ energy. 1) loses 2) gains change 3) does not C. The ice ______energy. 1) loses 2) gains 3) does not change D. The final temperature of the ice and liquid is 1) the same 26 2) different

SOLUTION CS 4 A. Ice cubes in a warm drink will 1) melt B. The liquid drink 1) loses energy. C. The ice 2) gains energy. D. The final temperature of the ice and liquid is 1) the same 27

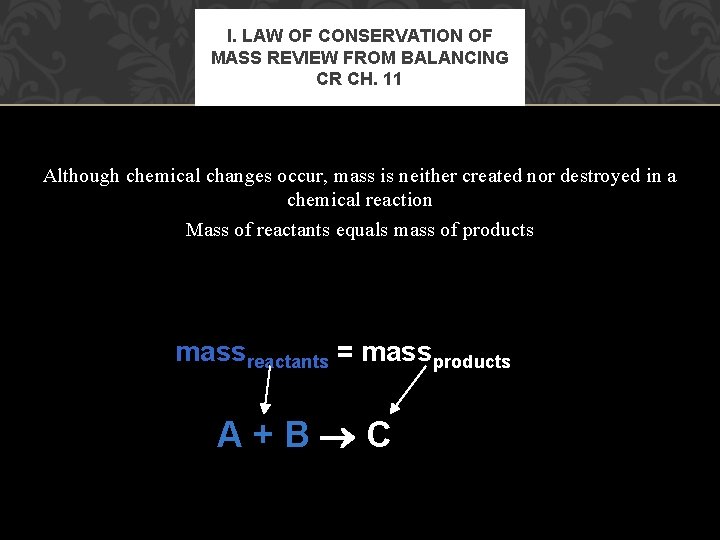
I. LAW OF CONSERVATION OF MASS REVIEW FROM BALANCING CR CH. 11 Although chemical changes occur, mass is neither created nor destroyed in a chemical reaction Mass of reactants equals mass of products massreactants = massproductsts A+B C

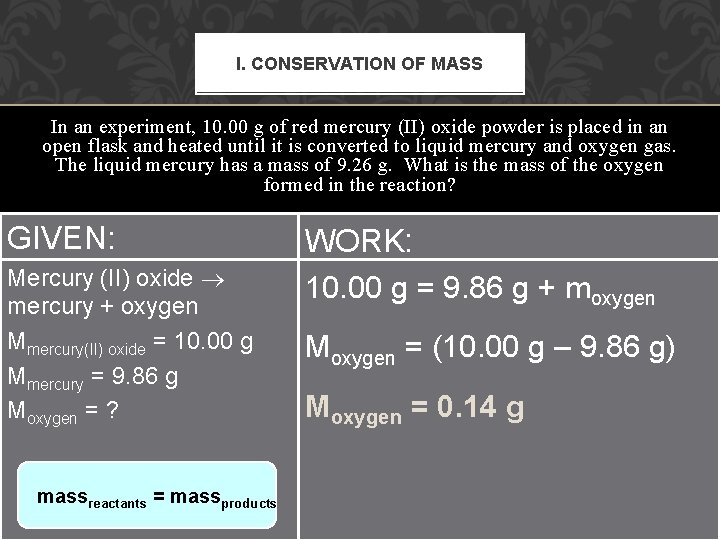
I. CONSERVATION OF MASS In an experiment, 10. 00 g of red mercury (II) oxide powder is placed in an open flask and heated until it is converted to liquid mercury and oxygen gas. The liquid mercury has a mass of 9. 26 g. What is the mass of the oxygen formed in the reaction? GIVEN: WORK: 10. 00 g = 9. 86 g + moxygen Mercury (II) oxide mercury + oxygen Mercury (II) oxide M mercury + oxygen Mmercury(II) oxide = 10. 00 g = (10. 00 g – 9. 86 oxygen Mmercury = 9. 86 g Mmercury(II) oxide = 10. 00 g Moxygen =? Mmercury = 9. 26 Moxygen = 0. 14 g Moxygen = ? massreactants = massproducts g)

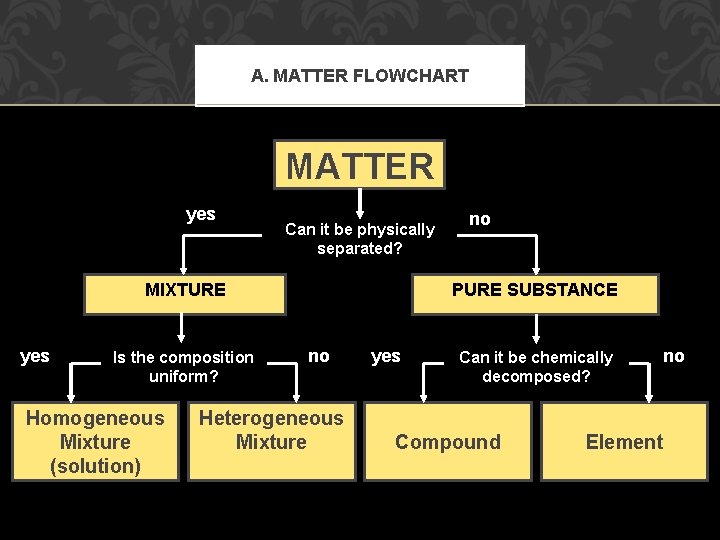
A. MATTER FLOWCHART MATTER yes Can it be physically separated? PURE SUBSTANCE MIXTURE yes Is the composition uniform? Homogeneous Mixture (solution) no no Heterogeneous Mixture yes Can it be chemically decomposed? Compound no Element
 Four states of matter
Four states of matter Solid liquid gas plasma
Solid liquid gas plasma Four states of matter
Four states of matter Four phases of matter
Four phases of matter Better matter
Better matter Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Tia chieu sa te
Tia chieu sa te đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới ưu thế lai là gì
ưu thế lai là gì Các môn thể thao bắt đầu bằng tiếng bóng
Các môn thể thao bắt đầu bằng tiếng bóng Tư thế ngồi viết
Tư thế ngồi viết Cái miệng nó xinh thế
Cái miệng nó xinh thế Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Tư thế ngồi viết
Tư thế ngồi viết Thế nào là giọng cùng tên? *
Thế nào là giọng cùng tên? * Chó sói
Chó sói Thẻ vin
Thẻ vin Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Hổ sinh sản vào mùa nào
Hổ sinh sản vào mùa nào Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Diễn thế sinh thái là
Diễn thế sinh thái là Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Lp html
Lp html Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 Lời thề hippocrates
Lời thề hippocrates Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Tư thế worms-breton
Tư thế worms-breton đại từ thay thế
đại từ thay thế