KINETIC THEORY Unit 7 Chemistry Langley Corresponds to





















- Slides: 39

KINETIC THEORY Unit 7 Chemistry Langley *Corresponds to Chapter 13 (pgs. 384 -409) in Prentice Hall Chemistry textbook

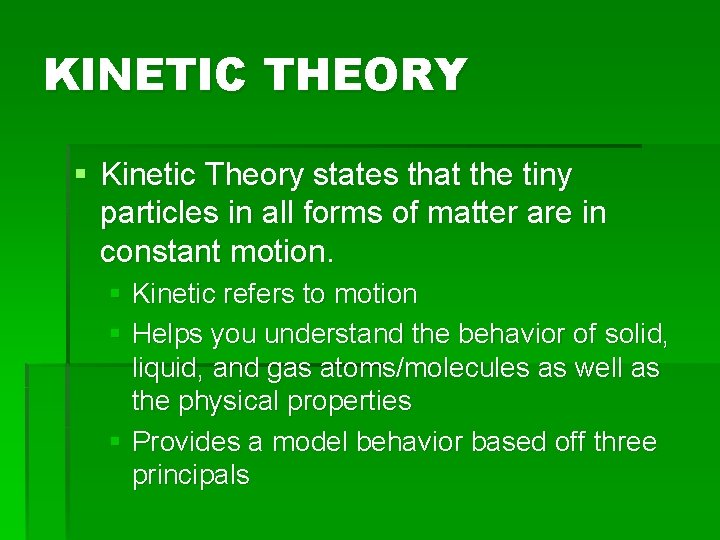
KINETIC THEORY § Kinetic Theory states that the tiny particles in all forms of matter are in constant motion. § Kinetic refers to motion § Helps you understand the behavior of solid, liquid, and gas atoms/molecules as well as the physical properties § Provides a model behavior based off three principals

KINETIC THEORY § 3 Principles of Kinetic Theory § All matter is made of tiny particles (atoms) § These particles are in constant motion § When particles collide with each other or the container, the collisions are perfectly elastic (no energy is lost)

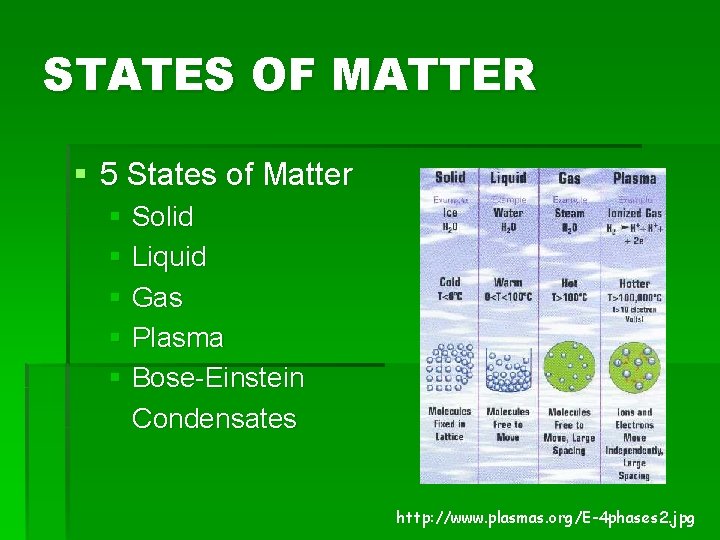
STATES OF MATTER § 5 States of Matter § Solid § Liquid § Gas § Plasma § Bose-Einstein Condensates http: //www. plasmas. org/E-4 phases 2. jpg

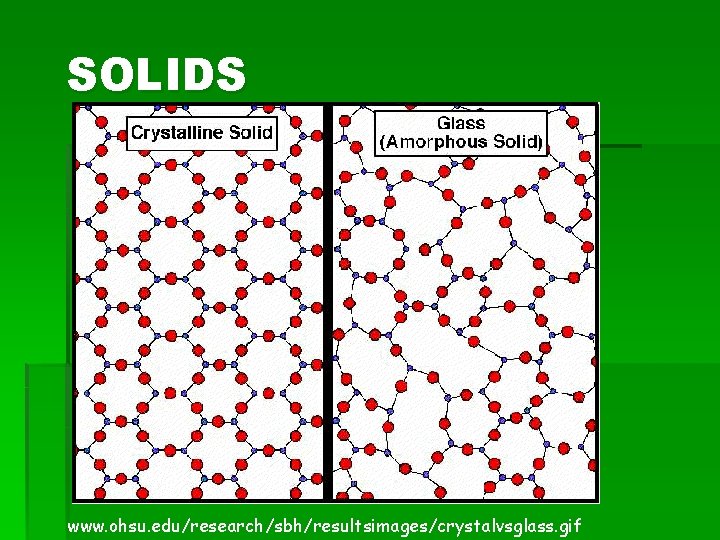
SOLIDS § § § Particles are tightly packed and close together Particles do move but not very much Definite shape and definite volume (because particles are packed closely and do not move) § Most solids are crystals § Crystals are made of unit cells (repeating patterns) § The shape of a crystal reflects the arrangement of the particles within the solid

SOLIDS § Unit cells put together make a crystal lattice (skeleton for the crystal) § Crystals are classified into seven crystal systems: cubic, tetragonal, orthorhombic, monoclinic, triclinic, hexagonal, rhombohedral § Unit cell crystal lattice solid

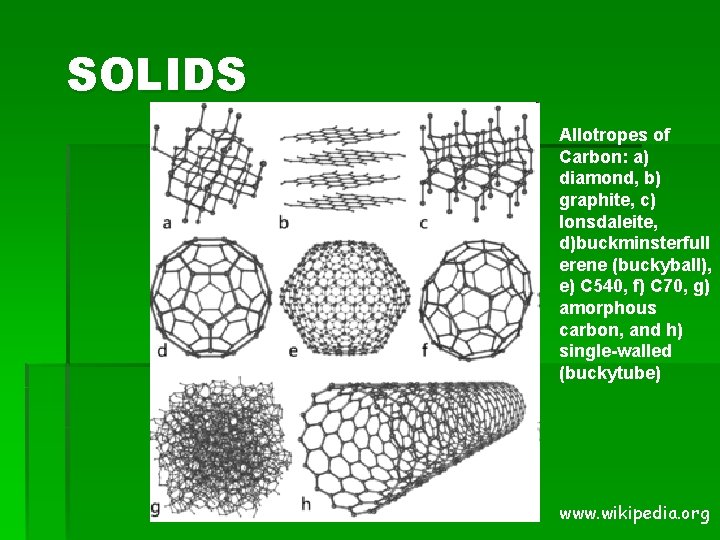
SOLIDS § Amorphous Solid: § A solid with no defined shape (not a crystal) § A solid that lacks an ordered internal structure § Examples: Clay, Play. Doh, Rubber, Glass, Plastic, Asphalt § Allotropes: § Solids that appear in more than one form § 2 or more different molecular forms of the same element in the same physical state (have different properties) § Example: Carbon § § § Powder = Graphite Pencil “lead” = graphite Hard solid = diamond

SOLIDS www. ohsu. edu/research/sbh/resultsimages/crystalvsglass. gif

SOLIDS Allotropes of Carbon: a) diamond, b) graphite, c) lonsdaleite, d)buckminsterfull erene (buckyball), e) C 540, f) C 70, g) amorphous carbon, and h) single-walled (buckytube) www. wikipedia. org

LIQUIDS § § § Particles are spread apart Particles move slowly through a container No definite shape but do have a definite volume Flow from one container to another Viscosity – resistance of a liquid to flowing § Honey – high viscosity § Water – low viscosity chemed. chem. purdue. edu/. . . /graphics

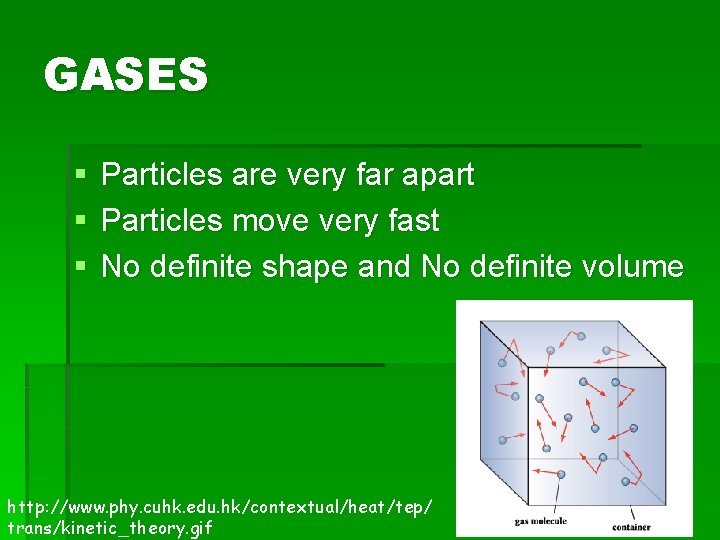
GASES § § § Particles are very far apart Particles move very fast No definite shape and No definite volume http: //www. phy. cuhk. edu. hk/contextual/heat/tep/ trans/kinetic_theory. gif

PLASMA § § § Particles are extremely far apart Particles move extremely fast Only exists above 3000 degrees Celsius Basically, plasma is a hot gas When particles collide, they break apart into protons, neutrons, and electrons § Occurs naturally on the sun and stars

BOSE-EINSTEIN CONDENSATE § § § Particles extremely close together Particles barely move Only found at extremely cold temperatures § Basically Bose-Einstein is a cold solid § Lowest energy of the 5 states/phases of matter

GASES AND PRESSURE § Gas pressure is the force exerted by a gas per unit surface area of an object § Force and number of collisions § When there are no particles present, no collisions = no pressure = vacuum § Atmospheric Pressure is caused by a mixuture of gases (i. e. the air) § Results from gravity holding air molecules downward in/on the Earth’s atmosphere; atmospheric pressure decreases with altitude, increases with depth § Barometers are devices used to measure atmospheric pressure (contains mercury) § Standard Pressure is average normal pressure at sea level § As you go ABOVE sea level, pressure is less § As you go BELOW sea level, pressure is greater

GASES AND PRESSURE § Standard Pressure Values § At sea level the pressure can be recorded as: § § § 14. 7 psi (pounds per square inch) 29. 9 in. Hg (inches of Mercury) 760 mm. Hg (millimeters of Mercury) 760 torr 1 atm (atmosphere) 101. 325 k. Pa (kilopascals) § All of these values are EQUAL to each other: § § 29. 9 in. Hg = 101. 325 k. Pa 760 torr = 760 mm. Hg 1 atm = 14. 7 psi and so on………. § Say hello to Factor Label Method!!!!!!

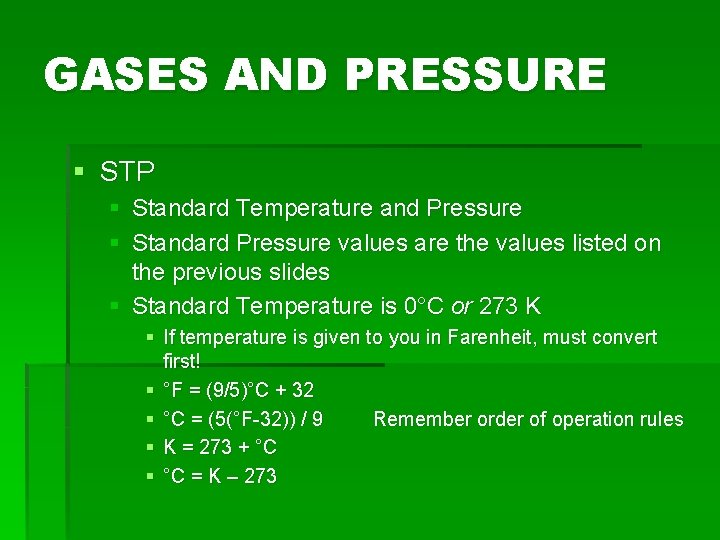
GASES AND PRESSURE § STP § Standard Temperature and Pressure § Standard Pressure values are the values listed on the previous slides § Standard Temperature is 0°C or 273 K § If temperature is given to you in Farenheit, must convert first! § °F = (9/5)°C + 32 § °C = (5(°F-32)) / 9 Remember order of operation rules § K = 273 + °C § °C = K – 273

GASES AND PRESSURE § Pressure Conversions § Example 1: 421 torr = ? Atm § Step 1: Write what you know § Step 2: Draw the fence and place the given in the top left § Step 3: Arrange what you know from step 1 such that the nondesired units canceling out so that you are only left with the units you want (i. e. atm) § Step 4: Solve § Step 5: Report final answer taking into account the appropriate significant figures

GASES AND PRESSURE § Pressure Conversions § Example 2: 32. 0 psi = ? torr

TEMPERATURE § Temperature is the measure of the average kinetic energy of the particles. § 3 Units for Temperature: § Celsius § Farenheit § Kelvin § Has an absolute zero § Absolute lowest possible temperature § All particles would completely stop moving § Temperature Conversions: § Example 1: Convert 35°C to °F § Example 2: Convert 300 Kelvin to °C

MEASURING PRESSURE § Manometers: § Measure pressure § 2 kinds: open and closed § Open Manometers: § Compare gas pressure to air pressure § Example: tire gauge § Closed Manometer: § Directly measure the pressure (no comparison) § Example: barometer

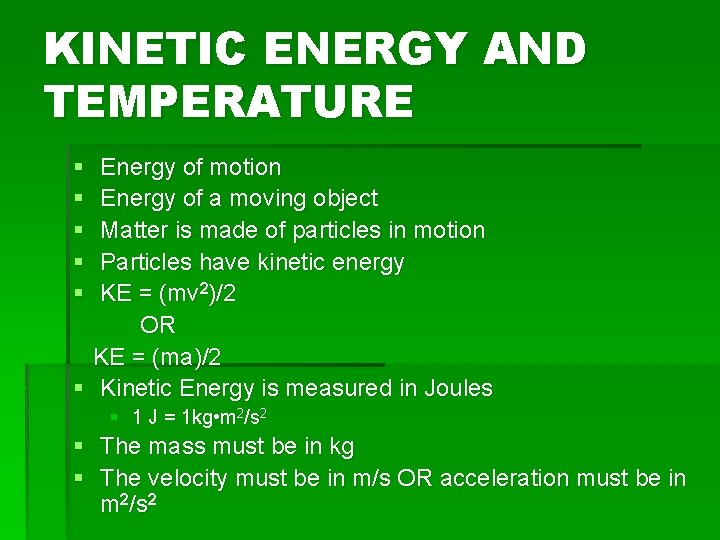
KINETIC ENERGY AND TEMPERATURE § § § Energy of motion Energy of a moving object Matter is made of particles in motion Particles have kinetic energy KE = (mv 2)/2 OR KE = (ma)/2 § Kinetic Energy is measured in Joules § 1 J = 1 kg • m 2/s 2 § The mass must be in kg § The velocity must be in m/s OR acceleration must be in m 2/s 2

KINETIC ENERGY AND TEMPERATURE § Calculate the KE of a car with a mass of 1500 kg and a speed of 50 m/s

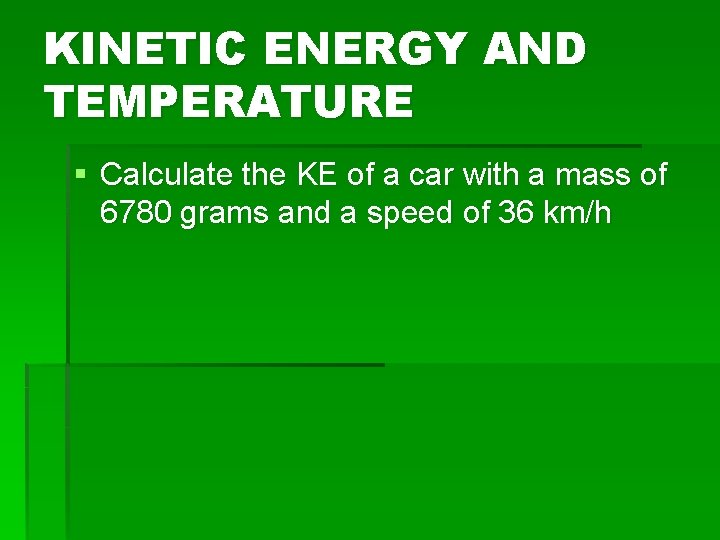
KINETIC ENERGY AND TEMPERATURE § Calculate the KE of a car with a mass of 6780 grams and a speed of 36 km/h

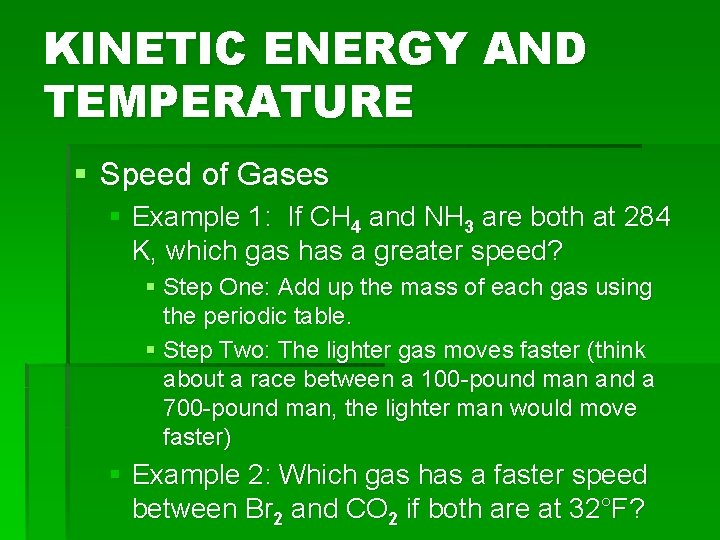
KINETIC ENERGY AND TEMPERATURE § Temperature-measure of the average kinetic energy of the particles § Kelvin Scale: § Has an absolute zero (0 K) § Absolute lowest possible temperature § In theory, all particles would completely stop moving § Speed of Gases: § If two gases have the same temperature (particles moving at the same speed) how can you tell which gas has a greater speed? § The only difference is mass! § To find mass, use the periodic table

KINETIC ENERGY AND TEMPERATURE § Speed of Gases § Example 1: If CH 4 and NH 3 are both at 284 K, which gas has a greater speed? § Step One: Add up the mass of each gas using the periodic table. § Step Two: The lighter gas moves faster (think about a race between a 100 -pound man and a 700 -pound man, the lighter man would move faster) § Example 2: Which gas has a faster speed between Br 2 and CO 2 if both are at 32°F?

TERMINOLOGY for PHASE CHANGES § Melting-commonly used to indicate changing from solid to liquid § Normal melting point-The temperature at which the vapor pressure of the solid and the vapor pressure of the liquid are equal § Freezing-Changing from a liquid to a solid § Melting and freezing occur at the same temperature § Liquifaction-Turning a gas to a liquid § Only happens in low temperature and high pressure situations

TERMINOLOGY for PHASE CHANGES § Difference in Gas and Vapor § Gas-state of matter that exists at normal room temperature § Vaport-produced by particles escaping from a state of matter that is normally liquid or solid at room temperature § Boiling-used to indicate changing from a liquid to a gas/vapor § Normal boiling point - temperature at which the vapor pressure of the liquid is equal to standard atmospheric pressure, which is 101. 325 k. Pa § Boiling point is a function of pressure. § At lower pressures, the boiling point is lower

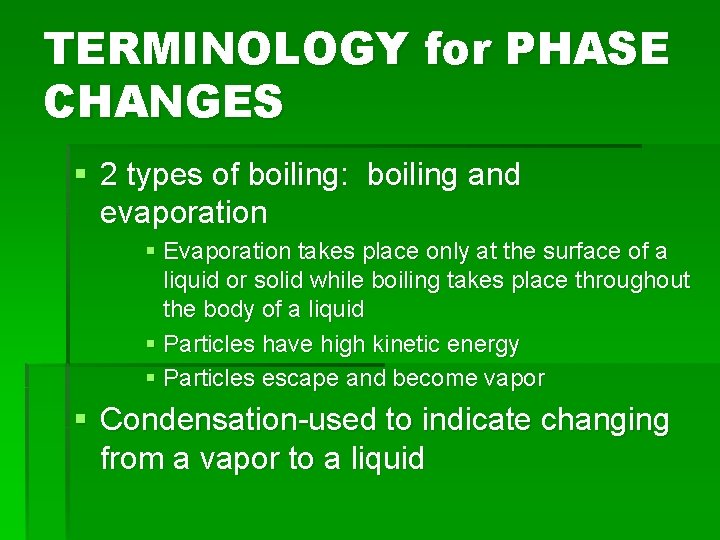
TERMINOLOGY for PHASE CHANGES § 2 types of boiling: boiling and evaporation § Evaporation takes place only at the surface of a liquid or solid while boiling takes place throughout the body of a liquid § Particles have high kinetic energy § Particles escape and become vapor § Condensation-used to indicate changing from a vapor to a liquid

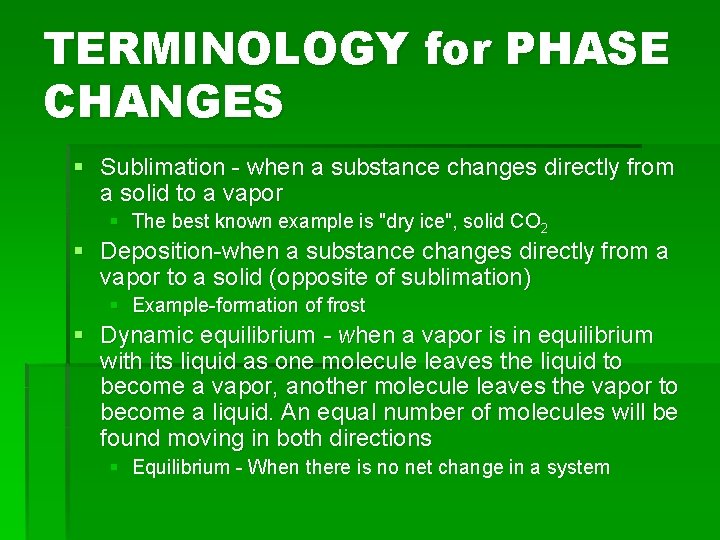
TERMINOLOGY for PHASE CHANGES § Sublimation - when a substance changes directly from a solid to a vapor § The best known example is "dry ice", solid CO 2 § Deposition-when a substance changes directly from a vapor to a solid (opposite of sublimation) § Example-formation of frost § Dynamic equilibrium - when a vapor is in equilibrium with its liquid as one molecule leaves the liquid to become a vapor, another molecule leaves the vapor to become a liquid. An equal number of molecules will be found moving in both directions § Equilibrium - When there is no net change in a system

TERMINOLOGY for PHASE CHANGES § Points to Know: § Melting Point-Temperature when solid turns to a liquid § Freezing Point-Temperature when liquid turns to a solid § Boling Point-Temperature when a liquid turns to a vapor § Doesn’t boil unitl vapor pressure coming off liquid is equal to the air pressure around it § Since air pressure changes with height, water does not always boil at 100°C § Condensing Point-Tempeature when vapor turns to liquid

ENTROPY § A measure of the disorder of a system § Systems tend to go from a state of order (low entropy) to a state of maximum disorder (high entropy) § Entropy of a gas is greater than that of a liquid; entropy of a liquid is greater than that of a solid § Solids=low entropy; plasma=high entropy § Entropy tends to increase when temperature increases § As substances change from one state to another, entropy may increase or decrease

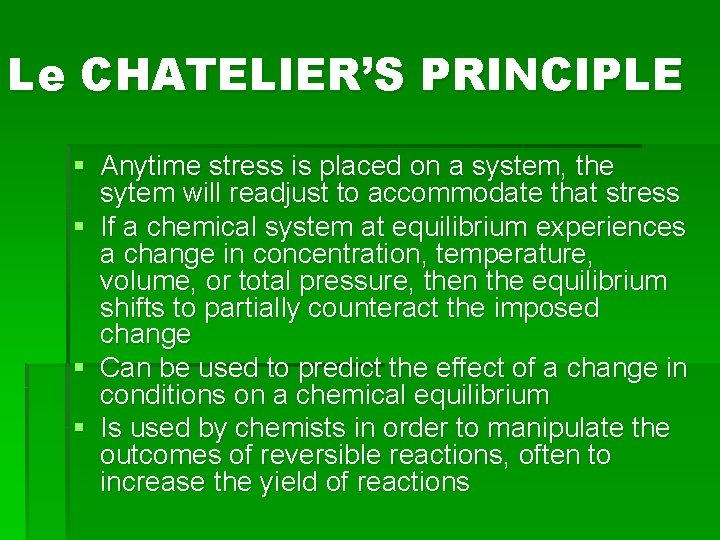
Le CHATELIER’S PRINCIPLE § Anytime stress is placed on a system, the sytem will readjust to accommodate that stress § If a chemical system at equilibrium experiences a change in concentration, temperature, volume, or total pressure, then the equilibrium shifts to partially counteract the imposed change § Can be used to predict the effect of a change in conditions on a chemical equilibrium § Is used by chemists in order to manipulate the outcomes of reversible reactions, often to increase the yield of reactions

Le CHATELIER’S PRINCIPLE § When liquids are heated (stress) they produce vapor particles (adjust) § When liquids are cooled (stress) the particles inside tighten to form a solid (adjust)

Le CHATELIER’S PRINCIPLE § Le Chatelier’s Principle explaining boiling and condensation using covered beaker partially filled with water § At a given temperature the covered beaker constitutes a system in which the liquid water is in equilibrium with the water vapor that forms above the surface of the liquid. § While some molecules of liquid are absorbing heat and evaporating to become vapor, an equal number of vapor molecules are giving up heat and condensing to become liquid. § If stress is put on the system by raising the temperature, then according to Le Châtelier's principle the rate of evaporation will exceed the rate of condensation until a new equilibrium is established

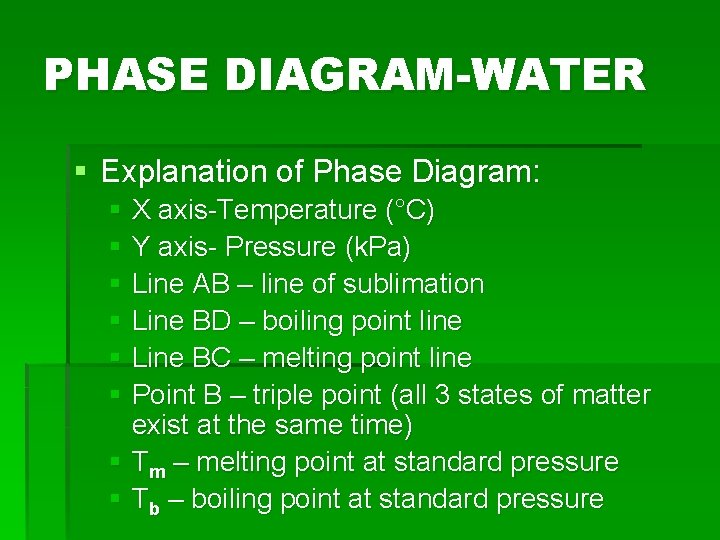
PHASE DIAGRAMS § A diagram showing the conditions at which substance exists as a solid, liquid, or vapor § Shows the temperature and pressure required for the 3 states of matter to exist § Conditions of pressure and temperature at which two phases exist in equilibrium are indicated on a phase diagram by a line separating the phases § Draw the phase diagram for water

PHASE DIAGRAM-WATER

PHASE DIAGRAM-WATER § Explanation of Phase Diagram: § X axis-Temperature (°C) § Y axis- Pressure (k. Pa) § Line AB – line of sublimation § Line BD – boiling point line § Line BC – melting point line § Point B – triple point (all 3 states of matter exist at the same time) § Tm – melting point at standard pressure § Tb – boiling point at standard pressure

HEAT in CHANGES of STATE § Energy Diagrams (also referred to as Heating Curves) § Graphically describes the enthalpy (the heat content of a system at sonstant pressure) changes that take place during phase changes § X axis is Energy (Heat supplied) § Y axis is Temperature

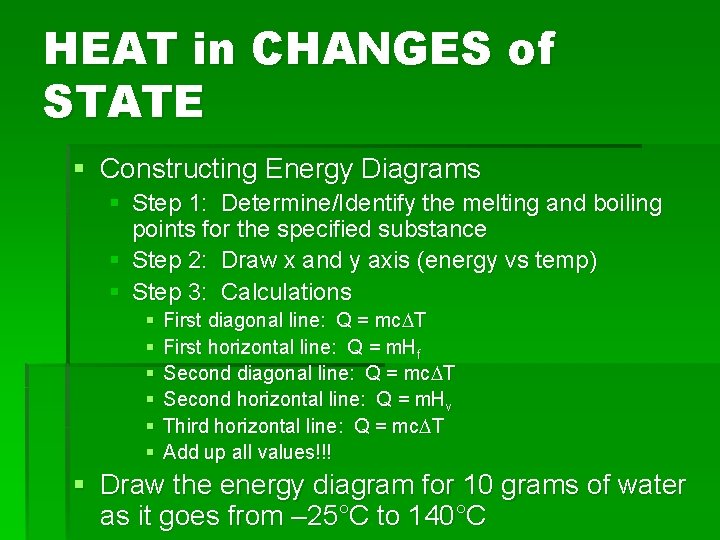
HEAT in CHANGES of STATE § Constructing Energy Diagrams § Step 1: Determine/Identify the melting and boiling points for the specified substance § Step 2: Draw x and y axis (energy vs temp) § Step 3: Calculations § § § First diagonal line: Q = mc. DT First horizontal line: Q = m. Hf Second diagonal line: Q = mc. DT Second horizontal line: Q = m. Hv Third horizontal line: Q = mc. DT Add up all values!!! § Draw the energy diagram for 10 grams of water as it goes from – 25°C to 140°C
 Fantail in ship
Fantail in ship Corresponds symbol
Corresponds symbol Maximum metal condition (mml) corresponds to the
Maximum metal condition (mml) corresponds to the Permeance of magnetic circuit corresponds to
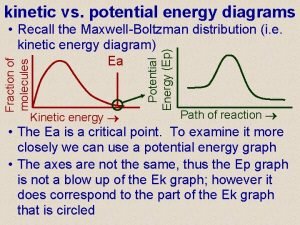
Permeance of magnetic circuit corresponds to Potential and kinetic energy diagram
Potential and kinetic energy diagram Langley hall laser login
Langley hall laser login Cardinal langley rc high school
Cardinal langley rc high school Cardinal langley holiday club
Cardinal langley holiday club Biography rosie langley wikipedia
Biography rosie langley wikipedia Edwina langley birmingham
Edwina langley birmingham Edwina langley birmingham
Edwina langley birmingham Edwina langley birmingham
Edwina langley birmingham Al langley nasa
Al langley nasa Nasa langley org chart
Nasa langley org chart Hayley langley
Hayley langley C. john langley, jr., ph.d.
C. john langley, jr., ph.d. C. john langley, jr., ph.d.
C. john langley, jr., ph.d. Short term rentals langley
Short term rentals langley Ib chemistry functional groups
Ib chemistry functional groups Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Unit 6 review questions
Unit 6 review questions Work done by a constant force
Work done by a constant force Si unit for kinetic energy
Si unit for kinetic energy Chapter 14 solids liquids and gases
Chapter 14 solids liquids and gases The kinetic theory of matter states that
The kinetic theory of matter states that Kinetic theory of matter definition
Kinetic theory of matter definition Define kinetic theory of matter
Define kinetic theory of matter An explanation of how particles in matter behave
An explanation of how particles in matter behave Kinetic molecular theory of gases
Kinetic molecular theory of gases The kinetic molecular theory
The kinetic molecular theory Kinetic molecular theory of liquids and solids
Kinetic molecular theory of liquids and solids Kinetic molecular theory of liquids
Kinetic molecular theory of liquids Kinetic energy molecular theory
Kinetic energy molecular theory Kinetic molecular theory def
Kinetic molecular theory def Particle theory freezing
Particle theory freezing Theory vs hypothesis
Theory vs hypothesis Kinetic theory of solids
Kinetic theory of solids Kinetic theory of gases
Kinetic theory of gases Charles law in terms of kinetic molecular theory
Charles law in terms of kinetic molecular theory Postulates for kinetic theory of gases
Postulates for kinetic theory of gases