States of Matter 1 States of Matter Most









- Slides: 14

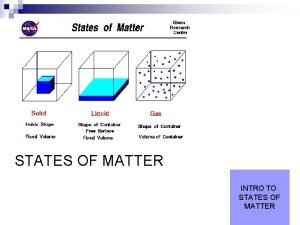
States of Matter 1

States of Matter Most common types: u. Solid u. Liquid u. Gas u. Plasma 2

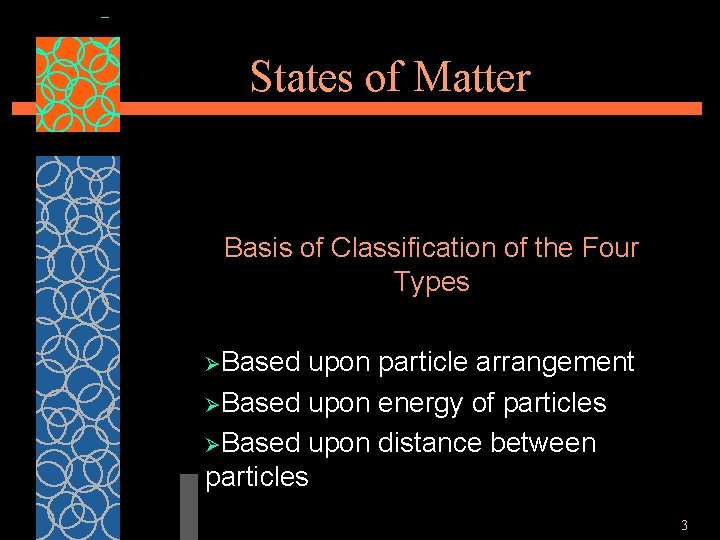
States of Matter Basis of Classification of the Four Types ØBased upon particle arrangement ØBased upon energy of particles ØBased upon distance between particles 3

States of Matter The Classification and Properties of Matter Depend Upon Microscopic Structure ØParticle arrangement ØParticle energy ØParticle to particle distance 4

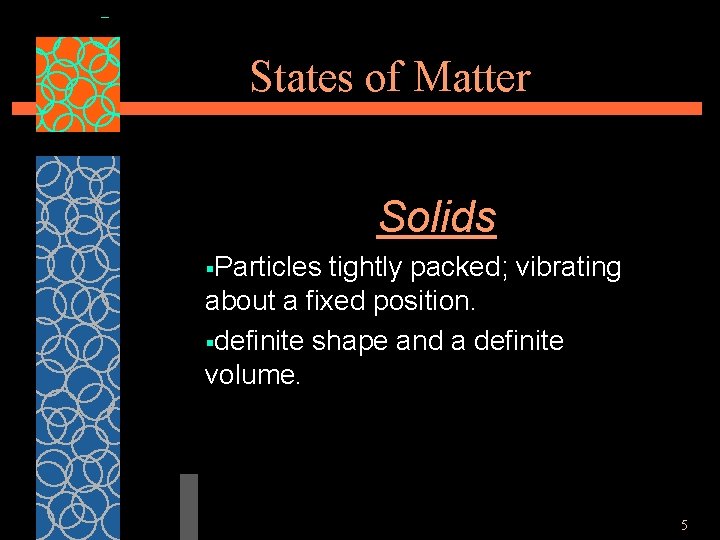
States of Matter Solids §Particles tightly packed; vibrating about a fixed position. §definite shape and a definite volume. 5

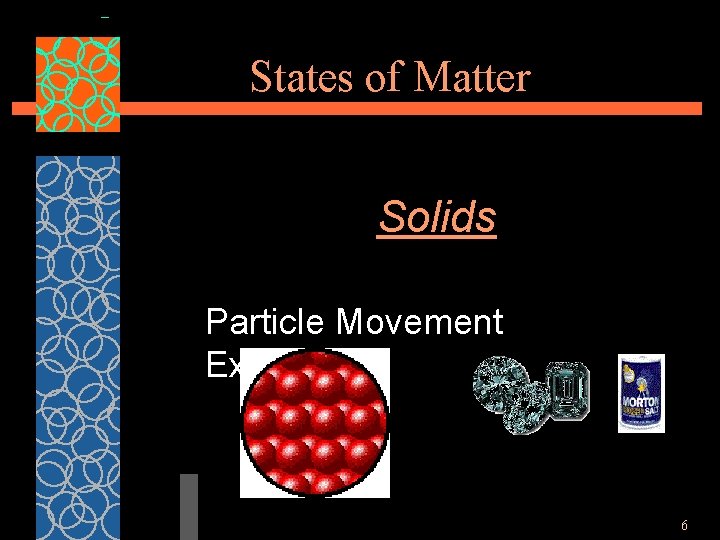
States of Matter Solids Particle Movement Examples 6

States of Matter Liquids §Particles: tightly packed, but are far enough apart to slide over one another § indefinite shape but a definite volume 7

States of Matter Liquids Particle Movement Examples 8

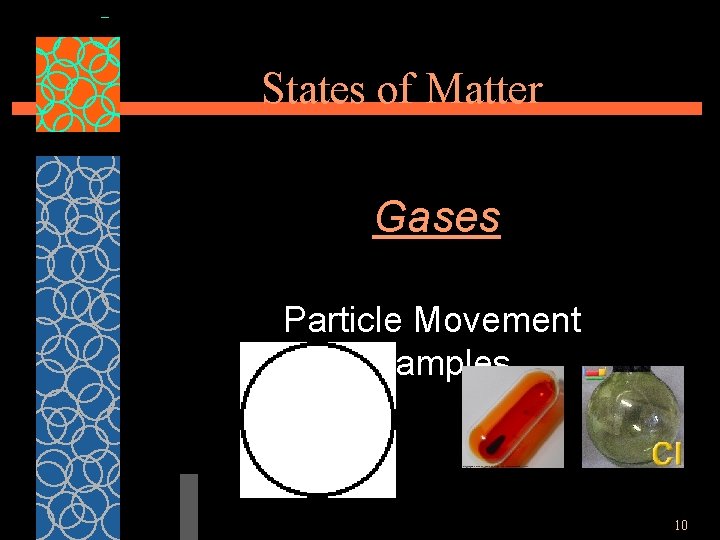
States of Matter Gases §particles are very far apart and move freely. § have an indefinite shape and an indefinite volume. 9

States of Matter Gases Particle Movement Examples 10

States of Matter Plasma § plasma is an ionized gas plasma is a very good conductor of electricity § §indefinite shape and an indefinite volume (like gases) 11

States of Matter Plasma Particles. 12

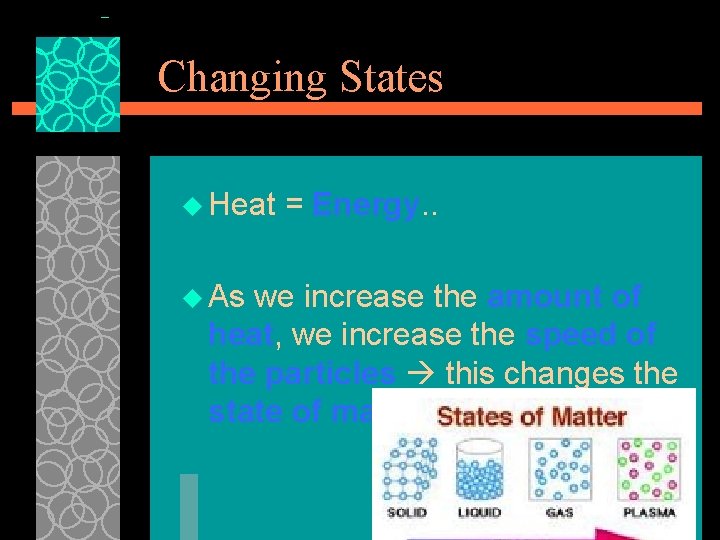
Changing States u Heat u As = Energy. . we increase the amount of heat, we increase the speed of the particles this changes the state of matter.

Additional States u Bose-Einstein u Condensate Fermionic Condensate Scientists create – atomic level (refrigerate particles – bosons – to very low temperature; wave of a speck of matter) Chumbler - Properties of Matter 14
 Solid
Solid Four states of matter
Four states of matter States of matter
States of matter Vocab
Vocab Four states of matter
Four states of matter Chapter 12 states of matter study guide
Chapter 12 states of matter study guide State of matter concept map
State of matter concept map Phet states of matter basics
Phet states of matter basics Uses of heat
Uses of heat How does the constitution guard against tyranny
How does the constitution guard against tyranny Five states of matter
Five states of matter Chapter 2 section 1 classifying matter answers
Chapter 2 section 1 classifying matter answers What were the 11 free states
What were the 11 free states Gray matter and white matter
Gray matter and white matter White matter of brain
White matter of brain