States of Matter States of matter Heating and








- Slides: 13

States of Matter States of matter Heating and Cooling

Lesson Objectives • I can understand how heating or cooling can change a material from a solid to a liquid and to a gas. • I can explore how different temperatures cause ice to melt at different rates. • I can investigate the melting and freezing temperature of a material.

Recap • In the previous two lessons we have seen that materials exist in one of three states; solid, liquid or gas. • Click on the picture to watch a video to remind us of this.

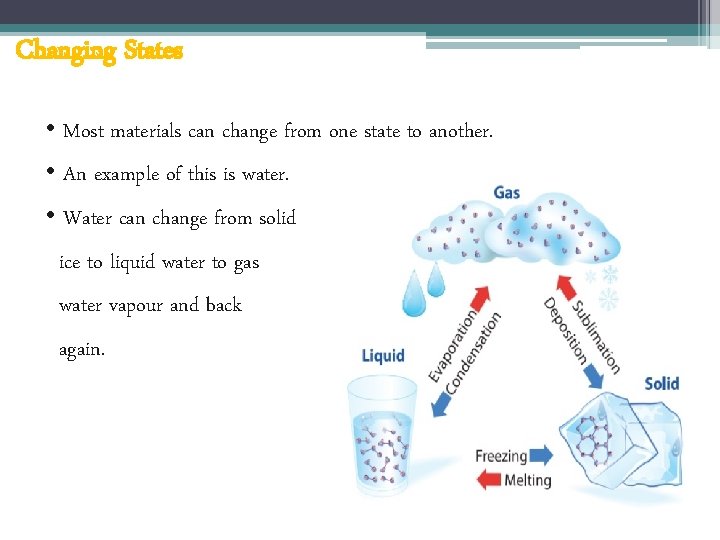
Changing States • Most materials can change from one state to another. • An example of this is water. • Water can change from solid ice to liquid water to gas water vapour and back again.

Changing States • To change a solid into a liquid or a liquid into a gas it needs to be heated. • To change a gas into a liquid or a liquid into a solid it needs to be cooled. An example of this is water.

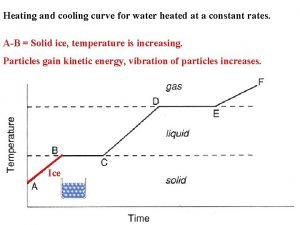
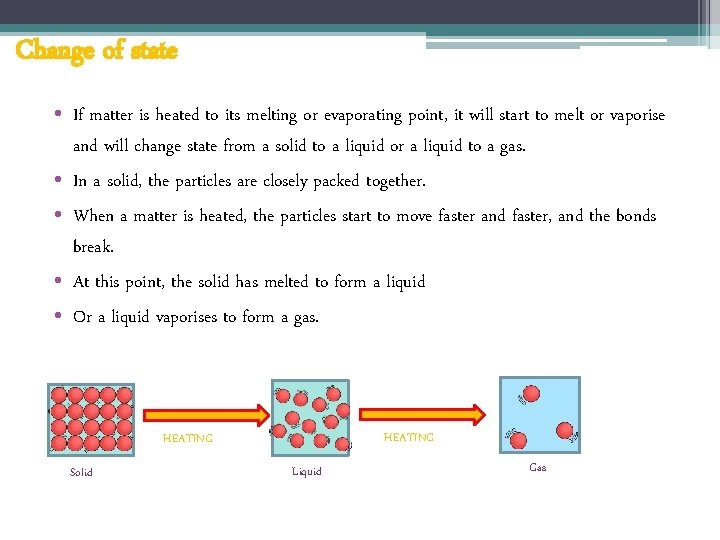
Change of state • If matter is heated to its melting or evaporating point, it will start to melt or vaporise and will change state from a solid to a liquid or a liquid to a gas. • In a solid, the particles are closely packed together. • When a matter is heated, the particles start to move faster and faster, and the bonds break. • At this point, the solid has melted to form a liquid • Or a liquid vaporises to form a gas. HEATING Solid Liquid Gas

Challenge 1 • • • In this challenge we will be looking at melting ice. We know that ice has a melting point, but where will ice melt fastest? We will place ice on a plate, in cool water and in hot water. Where will it melt fastest and slowest? Make a prediction then test your prediction.

Challenge 1 Results • Where did the ice melt first? • Was your prediction correct? • You should have found that it melted in the hot water first, because much more above the ice’s melting point. • Did the ice melt slowest in the cold water? • Why do you think this was?

Liquid to Solid • When a liquid turns into a solid it is called freezing. • When water freezes it turns into ice. • The temperature at which a liquid material freezes is called its freezing point. • Different materials have different freezing points.

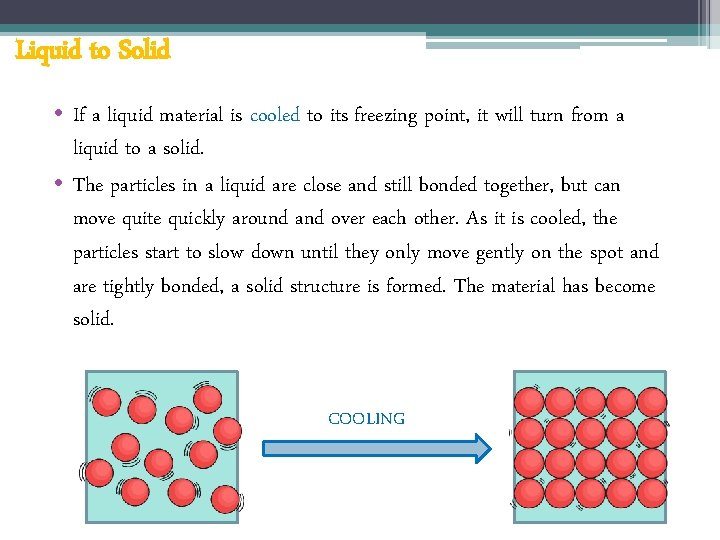
Liquid to Solid • If a liquid material is cooled to its freezing point, it will turn from a liquid to a solid. • The particles in a liquid are close and still bonded together, but can move quite quickly around and over each other. As it is cooled, the particles start to slow down until they only move gently on the spot and are tightly bonded, a solid structure is formed. The material has become solid. COOLING

Challenge 2 Activity 1 • In this activity we will be looking at cooling melted chocolate. • A member of staff will melt some chocolate and we will then use this to make cornflake cakes. • One set of cakes will be left out in the classroom and the others will be put in the fridge. • Can you predict which will become solid first?

Challenge 2 Results • Did the wax need to be put into the freezer to become solid? • What does this tell us about its freezing point? • Which set of chocolate cakes became solid first? • Why do you think this was the case? • How could we make the chocolate cakes go solid even quicker?

End of Session • If you have time at the end of the session then click on the picture below and watch a 4 minute video about solids, liquids and gases.
 Dielectric heating formula
Dielectric heating formula Physical behavior of matter
Physical behavior of matter Physical behavior of matter heating and cooling curves
Physical behavior of matter heating and cooling curves Plasma to gas
Plasma to gas Grey matter nervous system
Grey matter nervous system Brain falx
Brain falx Gray matter and white matter
Gray matter and white matter Frontal and parietal lobes
Frontal and parietal lobes Chapter 11 - states of matter: liquids and solids
Chapter 11 - states of matter: liquids and solids Definition of vocabulary
Definition of vocabulary Cooling load calculation software
Cooling load calculation software Heating and cooling curve
Heating and cooling curve Goddard heating and air
Goddard heating and air Unequal heating
Unequal heating