Chemistry Matter What is Matter Matter is anything
















- Slides: 19

Chemistry Matter

What is Matter? Matter is anything that has mass and takes up space. Can be a solid, liquid, gas, or plasma. We will be dealing with solids, liquids, and gases in this course.

We identify matter by it’s properties. Physical properties do not change the chemical nature of the matter. �They are broken down into two categories: �Extensive Physical Properties �Intensive Physical Properties Chemical properties change the chemical nature of the matter. �Ex) heat of combustion, reactivity with water, PH, and flammability, souring, rusting, etc

Extensive Physical Properties Depend on the amount of matter present. �Mass - A measurement of the amount of matter in a object (grams). �Weight - A measurement of the gravitational force of attraction of the earth acting on an object. �Volume - A measurement of the amount of space a substance occupies. �Length

Intensive Physical Properties Do Not depend on the amount of matter present. � Color � Odor � Luster - How shiny a substance is. � Malleability - The ability of a substance to be beaten into thin sheets. � Ductility - The ability of a substance to be drawn into thin wires. � Conductivity - The ability of a substance to allow the flow of energy or electricity. � Hardness - How easily a substance can be scratched. � Melting/Freezing Point - The temperature at which the solid and liquid phases of a substance are in equilibrium at atmospheric pressure. � Boiling Point - The temperature at which the vapor pressure of a liquid is equal to the pressure on the liquid (generally atmospheric pressure). � Density - The mass of a substance divided by its volume

So, what is a Physical Change? �Only the form of the substance changes �ice melting �water boiling �cutting a rope in two �grinding a piece of rock salt into a fine powder

What is a Chemical Change? �Substances are changed into different substances so the composition of the substance changes. �Explosion of fireworks �Burning paper �Leaves changing color in the fall

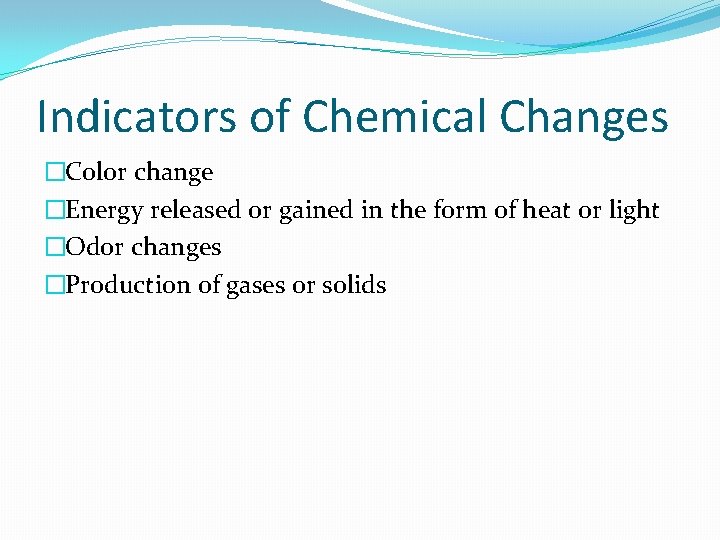
Indicators of Chemical Changes �Color change �Energy released or gained in the form of heat or light �Odor changes �Production of gases or solids

Still having trouble? Think about it this way… �Chemical changes are not easily reversed. �As wood burns, it turns into a pile of ashes and gases that rise into air. After the wood is burned, it cannot be restored to its original form as a log. �Physical changes can be reversed. �Think about ice for a moment. After ice melts into liquid water, you can refreeze it into solid ice if the temperature drops. Freezing and melting are physical changes.

Most important thing to remember Physical - the composition does not change. and Chemical - the composition changes.

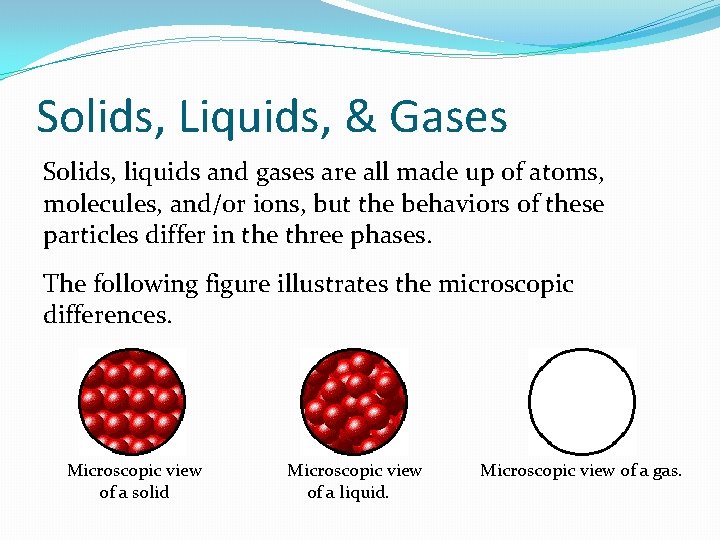
Solids, Liquids, & Gases Solids, liquids and gases are all made up of atoms, molecules, and/or ions, but the behaviors of these particles differ in the three phases. The following figure illustrates the microscopic differences. Microscopic view of a solid Microscopic view of a liquid. Microscopic view of a gas.

Note that: Particles in a: �gas are well separated with no regular arrangement. �liquid are close together with no regular arrangement. �solid are tightly packed, usually in a regular pattern.

Also note that: Particles in a: �gas vibrate and move freely at high speeds. �liquid vibrate, move about, and slide past each other. �solid vibrate (jiggle) but generally do not move from place to place.

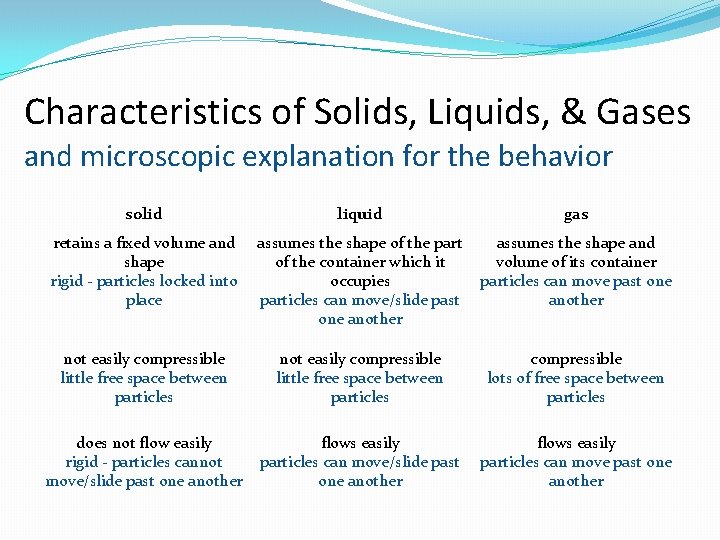
Characteristics of Solids, Liquids, & Gases and microscopic explanation for the behavior solid liquid gas retains a fixed volume and shape rigid - particles locked into place assumes the shape of the part of the container which it occupies particles can move/slide past one another assumes the shape and volume of its container particles can move past one another not easily compressible little free space between particles compressible lots of free space between particles does not flow easily rigid - particles cannot move/slide past one another flows easily particles can move past one another

Matter gets classified into 2 broad categories: Pure Substances And Mixtures

Pure Substances �Elements - all the same type of atom �Compounds - substances made from two or more different kinds of atoms.

Mixtures Homogeneous �Mixtures which are the same throughout with identical properties everywhere in the mixture. �Not easily separated. �This type of mixture is called a solution. A good example would be sugar dissolved in water or some type of metal alloy like the CROmium-MOLYbdenum steel used in many bike frames. Heterogeneous �Mixtures which have different properties when sampled from different areas. �Examples of this would be sand mixed with water or peanuts mixed with raisins.

Atoms vs. Molecules Atoms - the smallest piece of matter you can have that chemists can do reactions with is an atom. Each element has it's own type of atom. Molecules - two or more atoms bonded together with a covalent bond is called a molecule. a single atom (of an element) a molecule (of a compound) Note: Atoms don't have a color. The colors here are used to differentiate between kinds of atoms.

 Whats anything that has mass and takes up space
Whats anything that has mass and takes up space Matter is anything that
Matter is anything that Matter is anything that
Matter is anything that Is anything that has mass and volume
Is anything that has mass and volume Matter anything that
Matter anything that Is anything that has mass and takes up space.
Is anything that has mass and takes up space. Matter is anything that
Matter is anything that Anything that takes up space
Anything that takes up space Matter anything that
Matter anything that Matter anything that
Matter anything that Matter anything that
Matter anything that It is anything that has mass and occupies space
It is anything that has mass and occupies space Matter is anything that
Matter is anything that Ionized matter
Ionized matter What are the 7 diatomic elements
What are the 7 diatomic elements Mass vs weight
Mass vs weight Solubility defintion
Solubility defintion Use of density
Use of density Matter anything that
Matter anything that Matter is defined as anything that
Matter is defined as anything that