Matter Properties and Change What is Matter Matter











- Slides: 33

Matter: Properties and Change

What is Matter? Matter is anything that takes up space and/or has mass. Matter is made up of atoms and molecules.

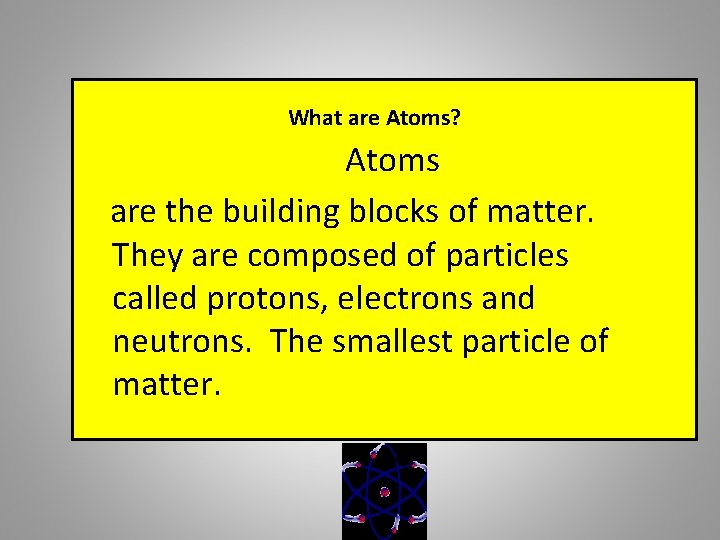
What are Atoms? Atoms are the building blocks of matter. They are composed of particles called protons, electrons and neutrons. The smallest particle of matter.

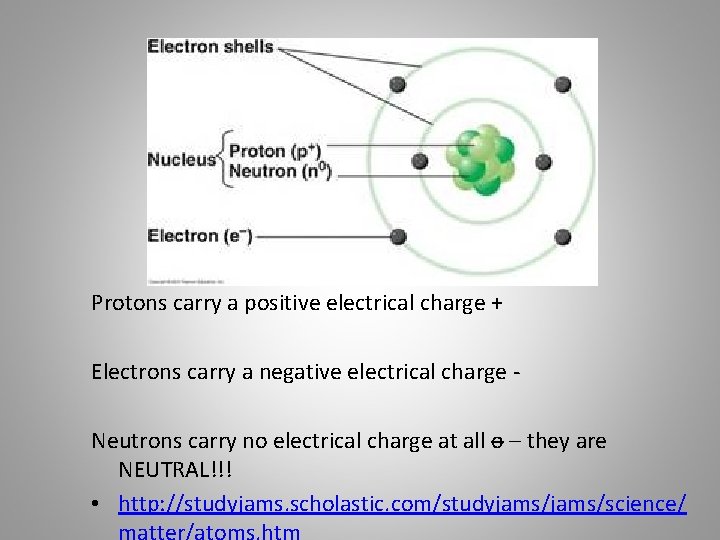
Protons carry a positive electrical charge + Electrons carry a negative electrical charge Neutrons carry no electrical charge at all o – they are NEUTRAL!!! • http: //studyjams. scholastic. com/studyjams/science/

What are Molecules? Molecules are a group of atoms bonded together.

What are Elements? . any material that cannot be broken down into more fundamental substances. Each element has only that specific type of atom. • http: //studyjams. scholastic. com/studyjams/science/matte r/elements-and-compounds. htm


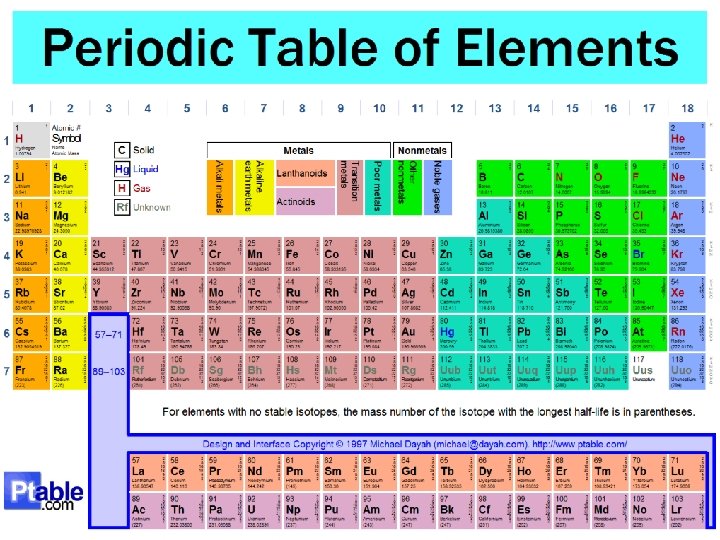
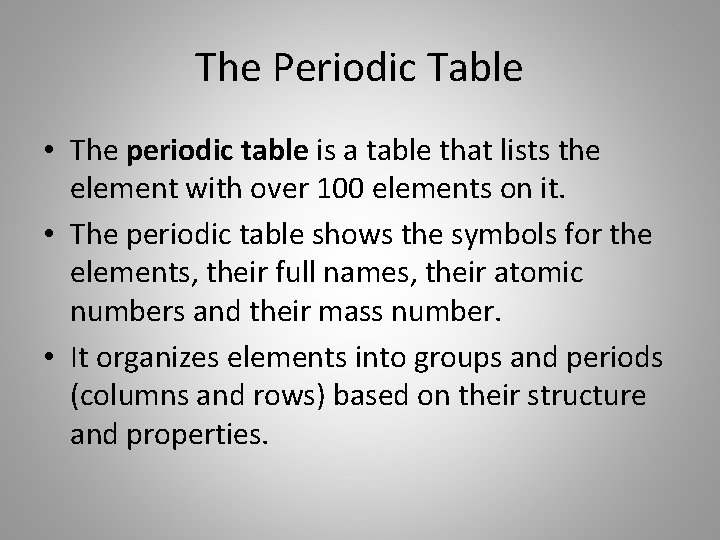
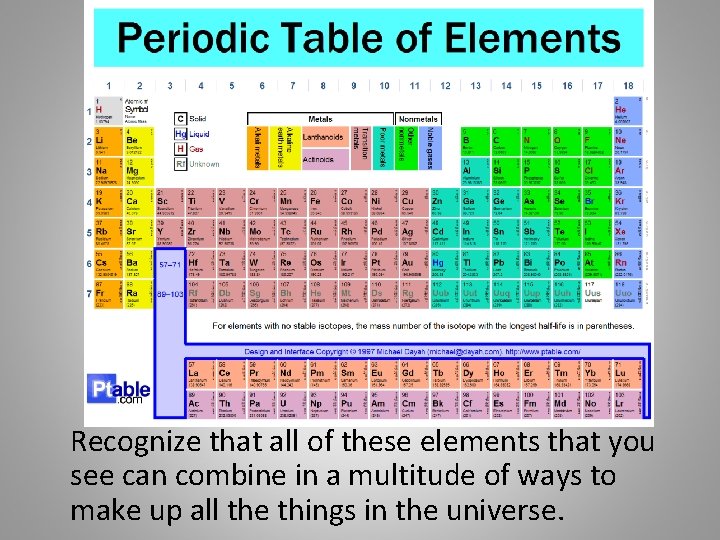
The Periodic Table • The periodic table is a table that lists the element with over 100 elements on it. • The periodic table shows the symbols for the elements, their full names, their atomic numbers and their mass number. • It organizes elements into groups and periods (columns and rows) based on their structure and properties.

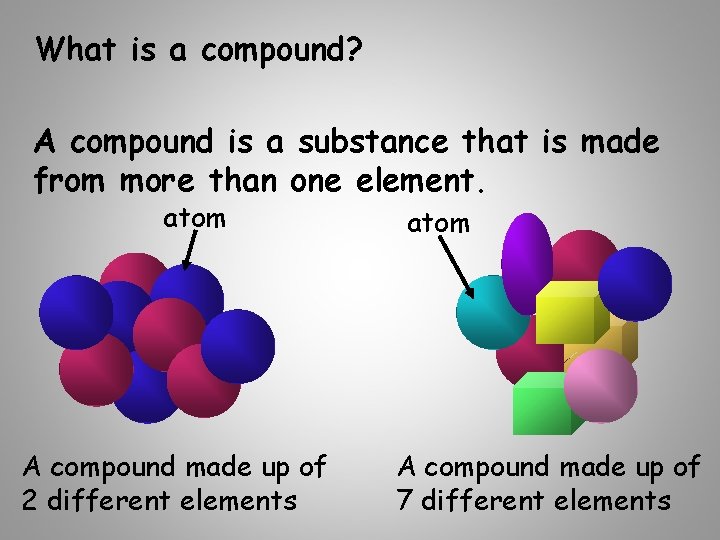
What is a compound? A compound is a substance that is made from more than one element. atom A compound made up of 2 different elements atom A compound made up of 7 different elements

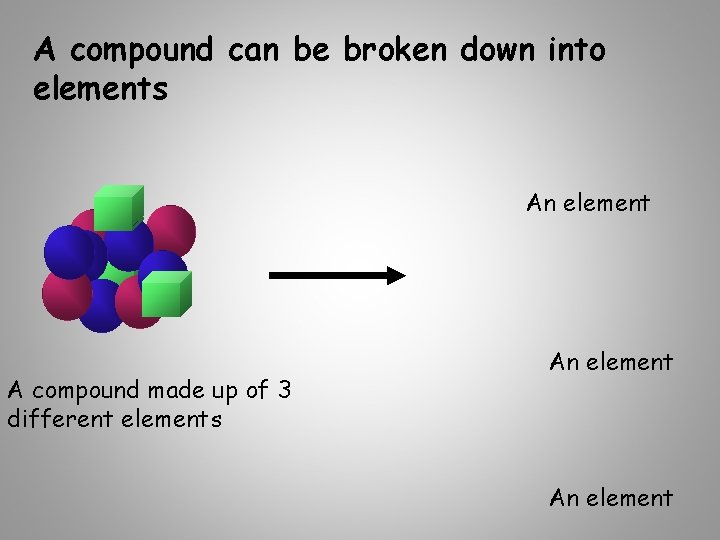
A compound can be broken down into elements An element A compound made up of 3 different elements An element

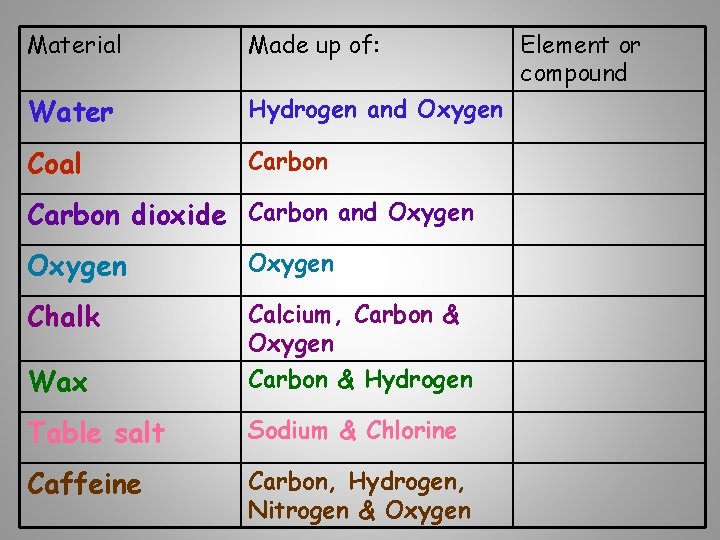
Material Made up of: Water Hydrogen and Oxygen Coal Carbon dioxide Carbon and Oxygen Chalk Wax Calcium, Carbon & Oxygen Carbon & Hydrogen Table salt Sodium & Chlorine Caffeine Carbon, Hydrogen, Nitrogen & Oxygen Element or compound

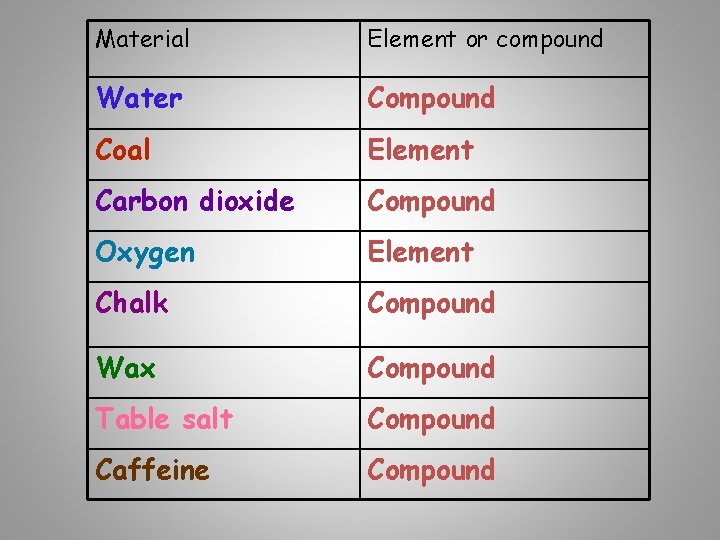
Material Element or compound Water Compound Coal Element Carbon dioxide Compound Oxygen Element Chalk Compound Wax Compound Table salt Compound Caffeine Compound

Mass is how much matter an object has *Mass is measured in grams (g) or kilograms (kg)

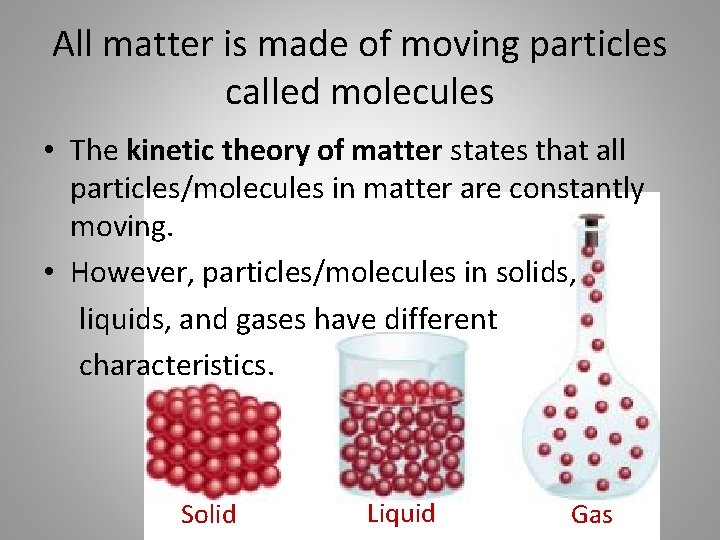
All matter is made of moving particles called molecules • The kinetic theory of matter states that all particles/molecules in matter are constantly moving. • However, particles/molecules in solids, liquids, and gases have different characteristics. Solid Liquid Gas

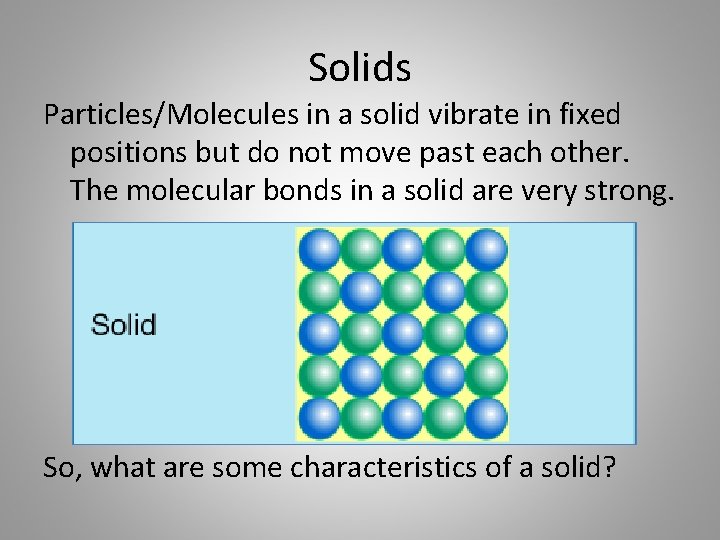
Solids Particles/Molecules in a solid vibrate in fixed positions but do not move past each other. The molecular bonds in a solid are very strong. So, what are some characteristics of a solid?

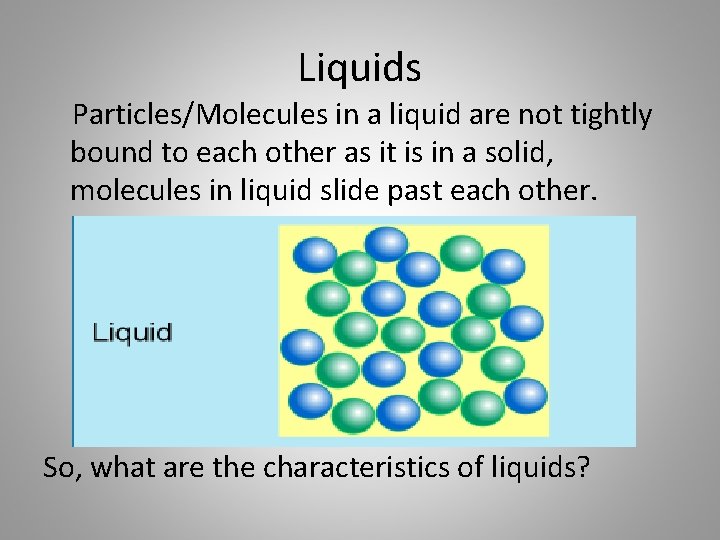
Liquids Particles/Molecules in a liquid are not tightly bound to each other as it is in a solid, molecules in liquid slide past each other. So, what are the characteristics of liquids?

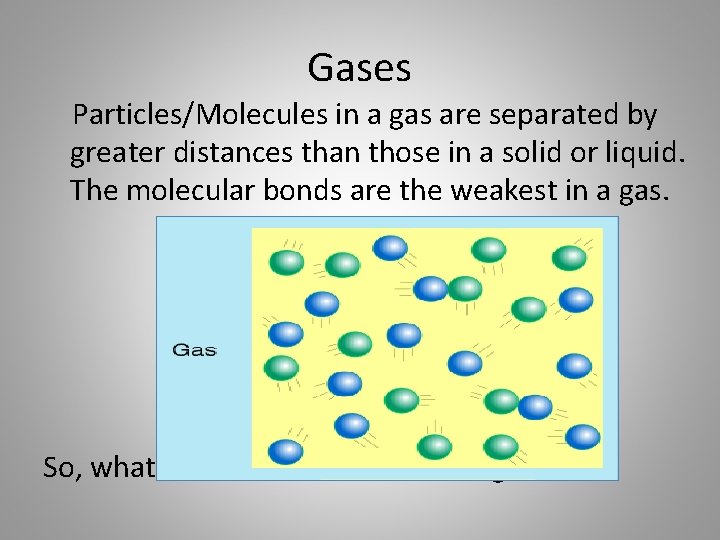
Gases Particles/Molecules in a gas are separated by greater distances than those in a solid or liquid. The molecular bonds are the weakest in a gas. So, what are the characteristics of gas?

FYI: There is a 4 th state of matter called plasma… Plasma is the most commonly seen state of matter in the universe. Plasma is similar to gas but portions are ionized. In an ordinary gas each atom contains an equal number of positive and negative charges; the positive charges in the nucleus are surrounded by an equal number of negatively charged electrons, and each atom is electrically "neutral. " A gas becomes a plasma when the addition of heat or other energy causes a significant number of atoms to release some or all of their electrons. The remaining parts of those atoms are left with a positive charge, and the detached negative electrons are free to move about. Those atoms and the resulting electrically charged gas are said to be "ionized. " When enough atoms are ionized to significantly affect the electrical characteristics of the gas, it is a plasma.

Recognize that all of these elements that you see can combine in a multitude of ways to make up all the things in the universe.

Temperature and Heat Unit B Chapter 2

Particles in a substance are not moving at the same speed and can change speeds.

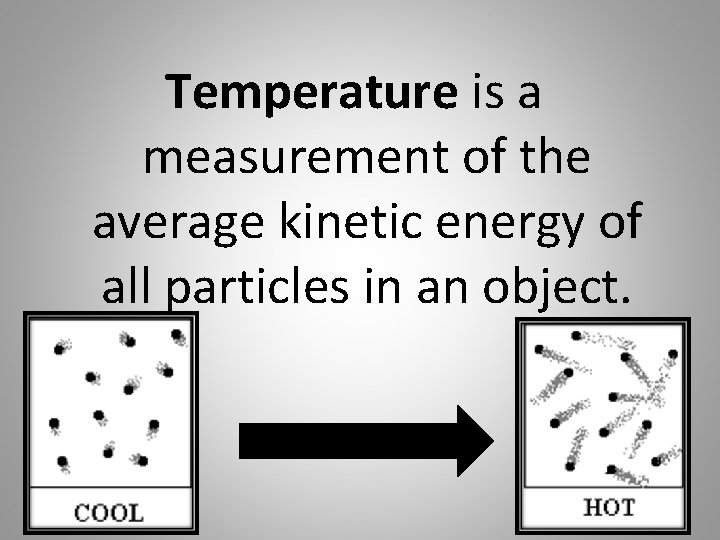
Temperature is a measurement of the average kinetic energy of all particles in an object.

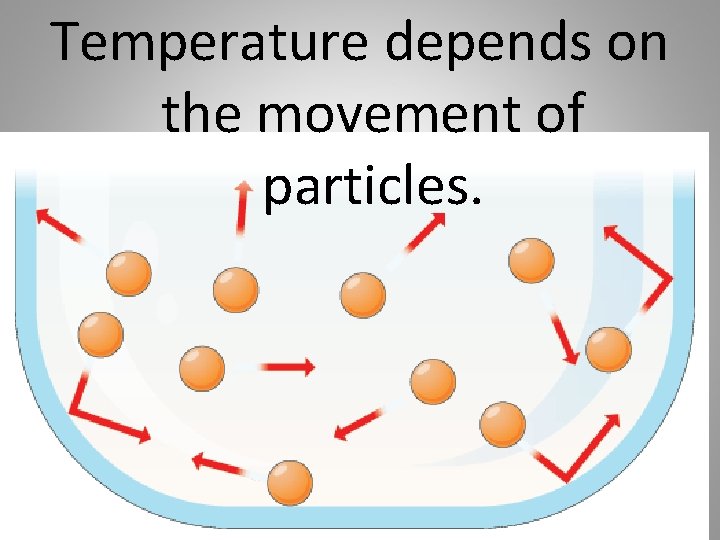
Temperature depends on the movement of particles.

Heat is a flow of energy due to temperature differences. And flows from higher temperatures to lower temperatures.

Now that’s HOT Ha ^ _ ^ ha

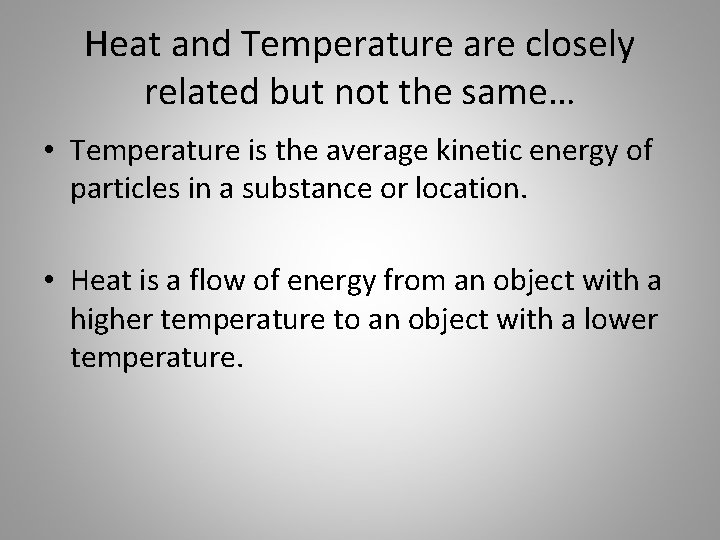
Heat and Temperature are closely related but not the same… • Temperature is the average kinetic energy of particles in a substance or location. • Heat is a flow of energy from an object with a higher temperature to an object with a lower temperature.

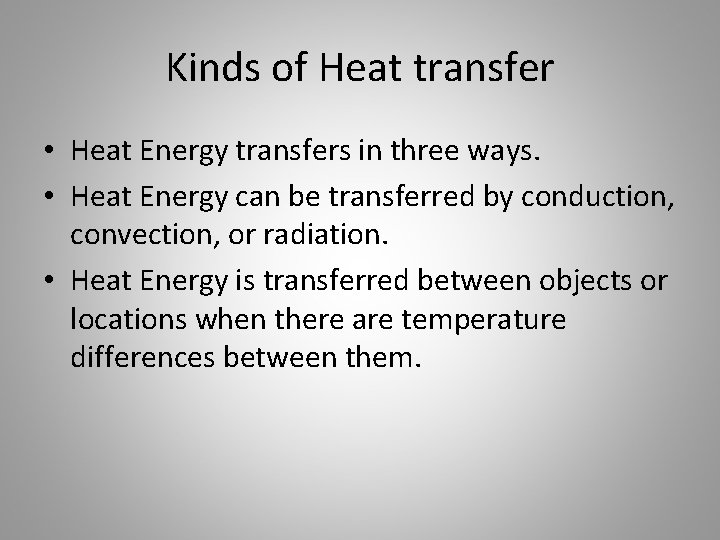
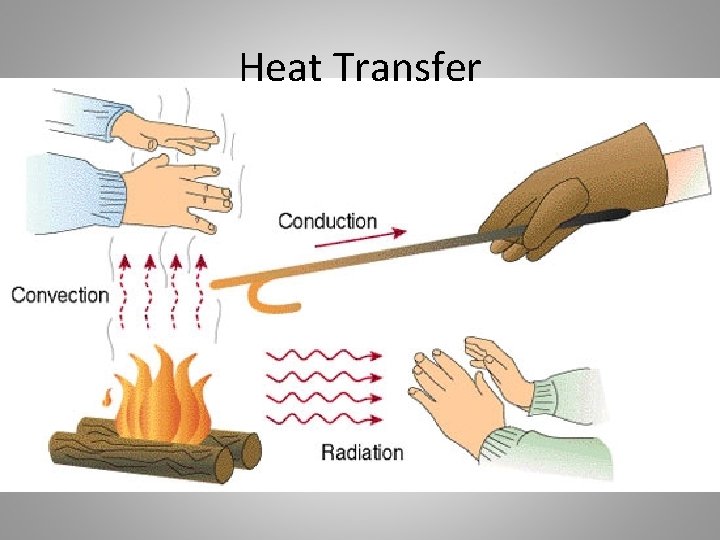
Kinds of Heat transfer • Heat Energy transfers in three ways. • Heat Energy can be transferred by conduction, convection, or radiation. • Heat Energy is transferred between objects or locations when there are temperature differences between them.

Conductors & Insulators • Materials that easily transfer energy are conductors; those that are poor conductors are called insulators. • Think about this question… • Why are pot and pan handles made out of plastic or wood? • Why is the pan made of metal? • Why are coolers made out of types of plastics? Good Conductors Metals Poor Conductors Wood Paper Plastic foam

Heat Transfer

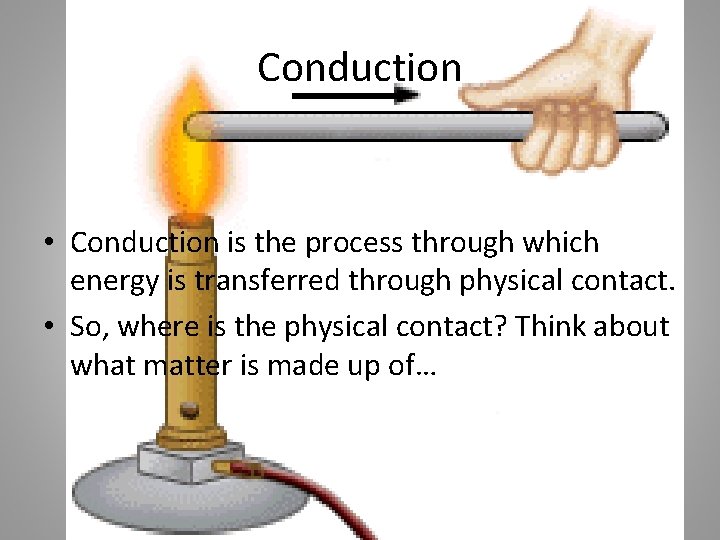
Conduction • Conduction is the process through which energy is transferred through physical contact. • So, where is the physical contact? Think about what matter is made up of…

Convection • Convection is the process that transfers energy in gases and liquids. • Hot air ____ and cold or cooler are _______. • Why do hot liquids and gases rise and cooler liquids and gases sink? Think about density… • When liquids and gases are heated, they become less dense and therefore will rise above any material that is more dense.

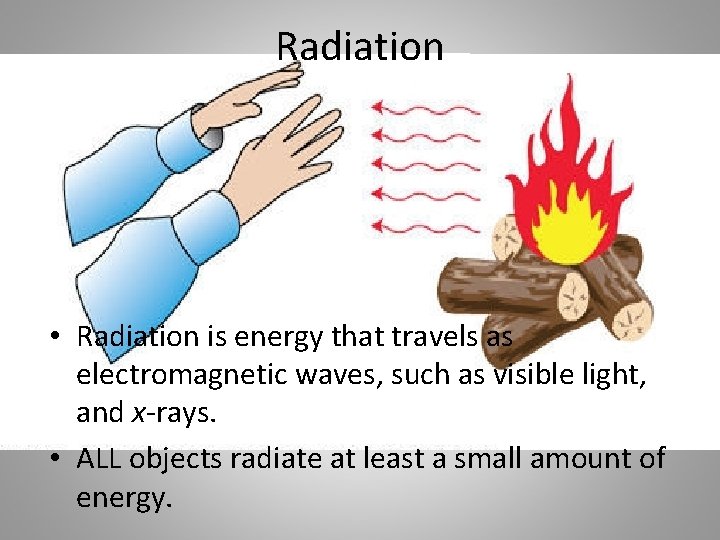
Radiation • Radiation is energy that travels as electromagnetic waves, such as visible light, and x-rays. • ALL objects radiate at least a small amount of energy.

Thermal Expansion - is a result of changes in temperature, matter changes in volume. Solids expand as the particles move ever so slightly farther apart.
 Intensive vs extensive properties
Intensive vs extensive properties Chemical and physical properties
Chemical and physical properties What are chemical changes
What are chemical changes Absolute change and relative change formula
Absolute change and relative change formula Differences between physical and chemical changes
Differences between physical and chemical changes Change in supply and change in quantity supplied
Change in supply and change in quantity supplied Chemical change and physical change
Chemical change and physical change Rocks change due to temperature and pressure change
Rocks change due to temperature and pressure change Physical change vs chemical change venn diagram
Physical change vs chemical change venn diagram First-order change and second-order change examples
First-order change and second-order change examples Classification and properties of matter
Classification and properties of matter Properties and changes of matter worksheet
Properties and changes of matter worksheet Matter and its properties
Matter and its properties The study of composition structure and properties
The study of composition structure and properties Matter-properties and changes answer key
Matter-properties and changes answer key Properties and characteristics of matter
Properties and characteristics of matter Big idea 9 changes in matter
Big idea 9 changes in matter Harmful and useful materials found at home
Harmful and useful materials found at home Grade 7 ns term 2
Grade 7 ns term 2 Chemistry matter and change chapter 7
Chemistry matter and change chapter 7 Chapter 10 the mole study guide
Chapter 10 the mole study guide Chapter 1 review matter and change
Chapter 1 review matter and change 1s 22 s22 p63 s23 p64 s2
1s 22 s22 p63 s23 p64 s2 Chemistry matter and change chapter 6
Chemistry matter and change chapter 6 Chemistry matter and change chapter 10
Chemistry matter and change chapter 10 Chemistry matter and change chapter 2 answer key
Chemistry matter and change chapter 2 answer key Unit 2 matter and change
Unit 2 matter and change Chapter 1 matter and change worksheet answers
Chapter 1 matter and change worksheet answers White matter of brain
White matter of brain Label the cranial dura septa and associated sinuses.
Label the cranial dura septa and associated sinuses. Gray matter and white matter
Gray matter and white matter Telencephalon
Telencephalon Examples for physical change
Examples for physical change Keep change change integers
Keep change change integers