Physical and Chemical Change Demo Physical Change Change











- Slides: 18
Physical and Chemical Change

Demo!

Physical Change • Change in state/phase • No new substance is formed • Only energy is changed – Cooling = energy is removed – Heating = energy is added • Usually reversible.

Physical Change examples Water evaporating (liquid to gas) Ice melting (solid to liquid) Plastic melting (solid to liquid) Paper ripping (solid to solid) BUT no new substance is formed. • Breaking a pencil – you have two pencils. Still no new substances are formed • Breaking a pen, you have no pen. Still no need substances are formed. • •

Chemical Change • New substance is made • Clues that a chemical change has occurred – Colour change – Heat or light is produced – Bubbles of gas is formed (not boiling) – Solid material may be formed in a solid (precipitate) – The change is difficult to REVERSE

Chemical Change example • • Acid and base reacting (forms water and salt) Burning something Explosions Digestion

Video!

Change is everywhere Video

Chemical change and physical change • When hydrogen gas (H 2) and oxygen gas (O 2) react, they form water. This is a chemical change • When water is heated up, it becomes a gas, but are still water molecules. This is a physical change.


Molecules during phase change • Molecules are always in motion • As substances are heated up – Amount of energy present increases • As substances are cooled – Amount of energy present decreases

Kinetic energy (molecule) • Molecules possess three types of kinetic energy • Rotational – where a molecule rotates about itself or an axis • Vibrational energy – changing bond length • Translational energy – energy that causes a molecule to move fast or slow in space

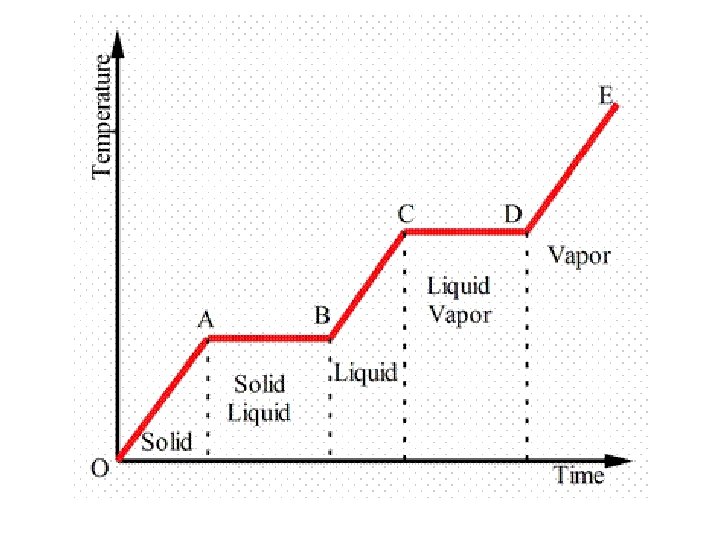
Pg 62 for diagram

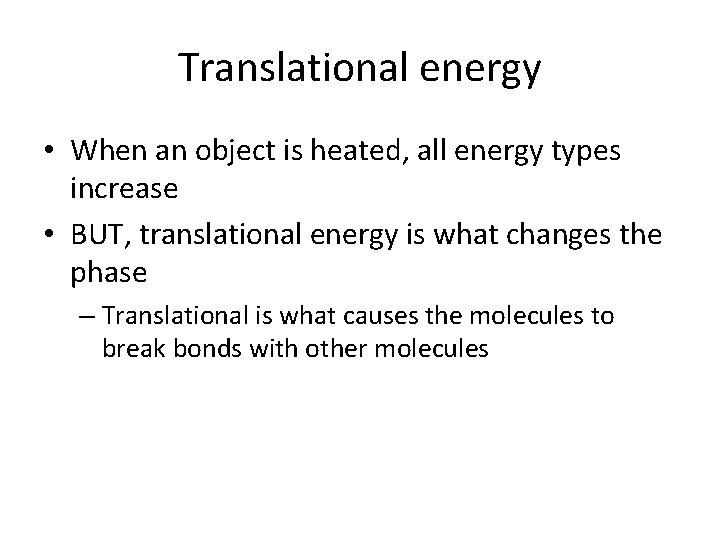
Translational energy • When an object is heated, all energy types increase • BUT, translational energy is what changes the phase – Translational is what causes the molecules to break bonds with other molecules

Simulation

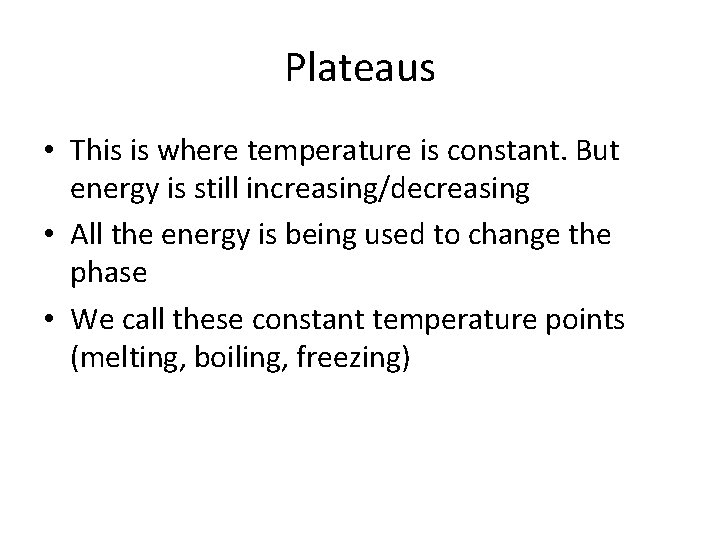
Plateaus • This is where temperature is constant. But energy is still increasing/decreasing • All the energy is being used to change the phase • We call these constant temperature points (melting, boiling, freezing)

NOTE!!! • Phase change is only a PHYSICAL CHANGE! • Plateaus and straight sloped lines are only present in PURE SUBSTANCES • In a mixture, you get wavy and jagged lines instead. As each substance in the mixture has their own phase change temperature.

Homework • Page 61 #59 -60 • Heath Review worksheet • Phase Change exercise on graphing
 What is a physical change
What is a physical change Differences between physical and chemical changes
Differences between physical and chemical changes Which is an example of a physical change
Which is an example of a physical change Whats the difference between chemical and physical change
Whats the difference between chemical and physical change Is painting a wall a physical change
Is painting a wall a physical change Spare change physical versus chemical change
Spare change physical versus chemical change How does a physical change differ from a chemical change? *
How does a physical change differ from a chemical change? * What is an example of physical change
What is an example of physical change Is paint drying a chemical change
Is paint drying a chemical change Chemical change grade 10
Chemical change grade 10 Whats the difference between chemical and physical change
Whats the difference between chemical and physical change Mixing red and green marbles physical change
Mixing red and green marbles physical change Differentiate between physical and chemical change
Differentiate between physical and chemical change Styrofoam and acetone chemical or physical change
Styrofoam and acetone chemical or physical change Class 7 chemical and physical change
Class 7 chemical and physical change Undesirable change
Undesirable change Baking cookies chemical or physical change
Baking cookies chemical or physical change Is aluminum foil cut in half a physical or chemical change
Is aluminum foil cut in half a physical or chemical change Is separating sand from gravel physical or chemical
Is separating sand from gravel physical or chemical