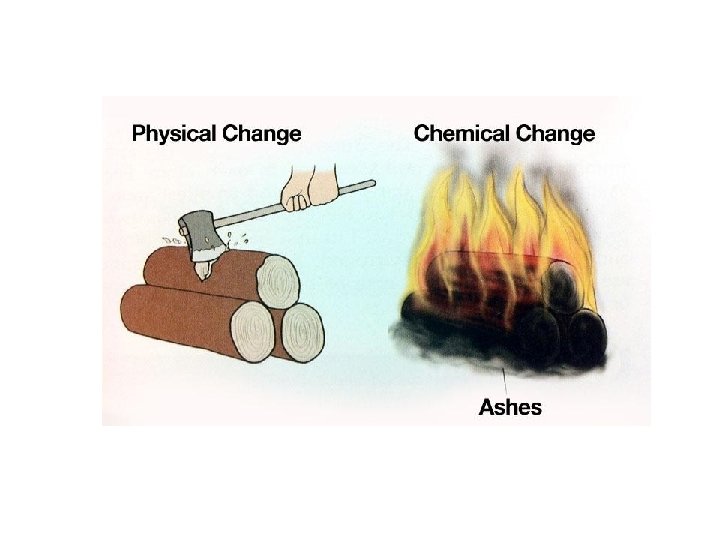
Changes Physical Chemical Physical Change A physical change




- Slides: 13

Changes Physical & Chemical

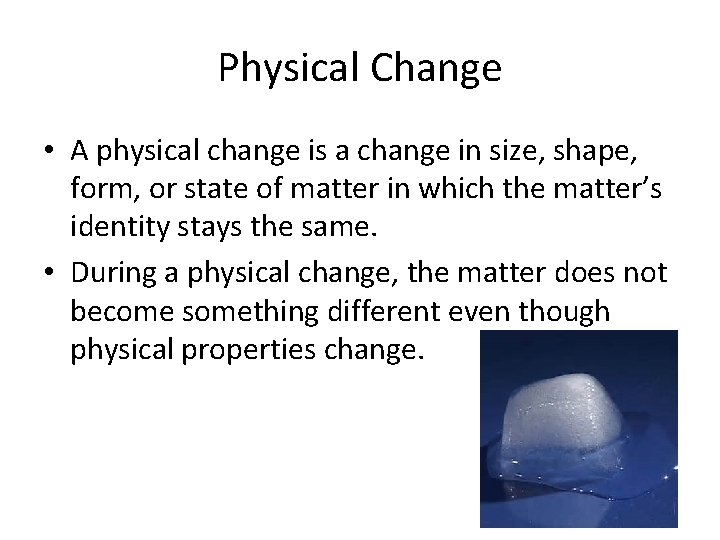
Physical Change • A physical change is a change in size, shape, form, or state of matter in which the matter’s identity stays the same. • During a physical change, the matter does not become something different even though physical properties change.

Change in Shape and Size • Chewing food – Breaking it down into smaller pieces • Pouring a liquid from container to container • Folding clothes/paper/etc Changes in shape and size are physical changes. The identity of the matter has not changed.

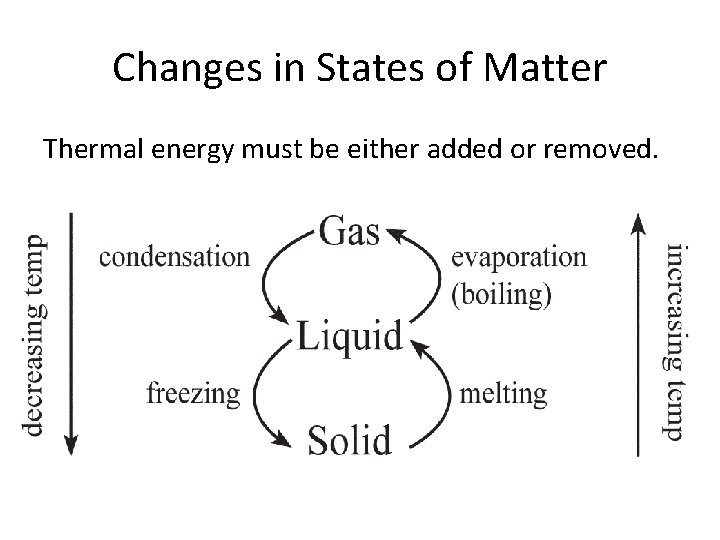
Changes in States of Matter Thermal energy must be either added or removed.

Adding Thermal Energy • When thermal energy is added to a solid, the particles in the solid move faster and faster, and the temperature increases. As the particles move faster, they are more likely to overcome the attractive forces that hold them tightly together. When this happens the solid has reached its melting point and changes from a solid to a liquid. • Adding more thermal energy will make the liquid change to a gas. This is the liquid’s boiling point.

Removing Thermal Energy • Removing thermal energy will slow down a materials particles. – Condensation: when a gas becomes a liquid – Freezing: when the particles move so slow their attraction holds them together tightly. This is when a liquid becomes a solid – Deposition: the change from a gas directly to a solid. – Sublimation: the change from a solid directly to a gas

Dissolving -Mixed evenly throughout a substance: salt water -Usually easy to reverse Evaporation Salt water Salt particles, no water

Conservation of Mass • The particles in matter that are present before a physical change are the same as those present after the physical change. The total mass before and after a change are the same according to the Conservation of Mass. • Let’s do the Lab page 367 and see this for ourselves!

Chemical Properties and Changes • Chemical Properties: are characteristics of matter that can be observed as it changes to a different type of matter. • Watch What happen to the paper? Can I get it back? Where did the paper go? • The ability of paper to burn is a chemical property of paper.

Chemical Changes A chemical change is a change in matter in which the substances that make up the matter change into other substances with new physical and chemical properties. Sometimes you can observe clues that a chemical change has occurred: bubbles, energy, odor, color

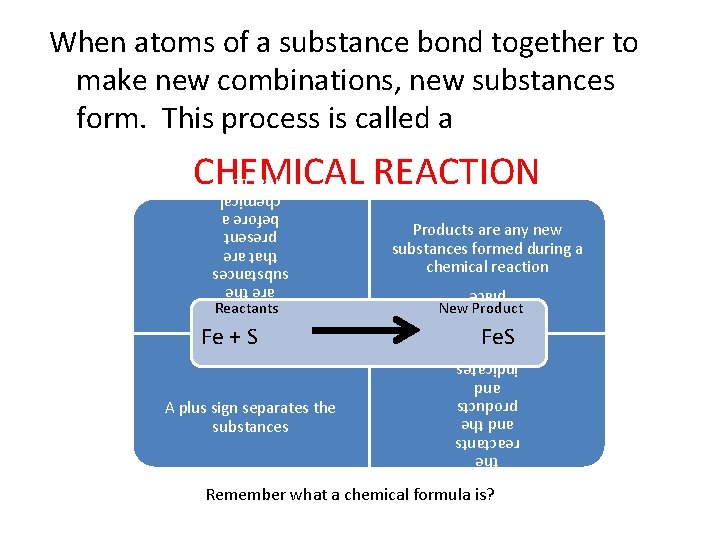
When atoms of a substance bond together to make new combinations, new substances form. This process is called a CHEMICAL REACTION Fe + S A plus sign separates the substances The arrow is read as “yields. ” It separates the reactants and the products and indicates that a reaction has taken place Reactants are the substances that are present before a chemical reaction Reactants Products are any new substances formed during a chemical reaction New Product Fe. S Remember what a chemical formula is?

Balancing Chemical Equations • Remember that the total mass before and after a change must be equal. Therefore, in a chemical equation, the number of atoms of each element before a reaction must equal the number of atoms of each element after the reaction. This is also the CONSERVATION OF MASS

Rate of Chemical Reactions • A higher temperature usually increases the rate of reaction. • Concentration is the amount of substance in a certain volume. More concentration/more particles • Surface area also affects reaction rate if at least one reactant is a solid. Bigger pieces usually take longer to react than smaller ones