Chapter 1 Matter Measurements Calculations 1 4 Classification








- Slides: 22

Chapter 1 Matter, Measurements, & Calculations 1. 4 Classification of Matter Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 1

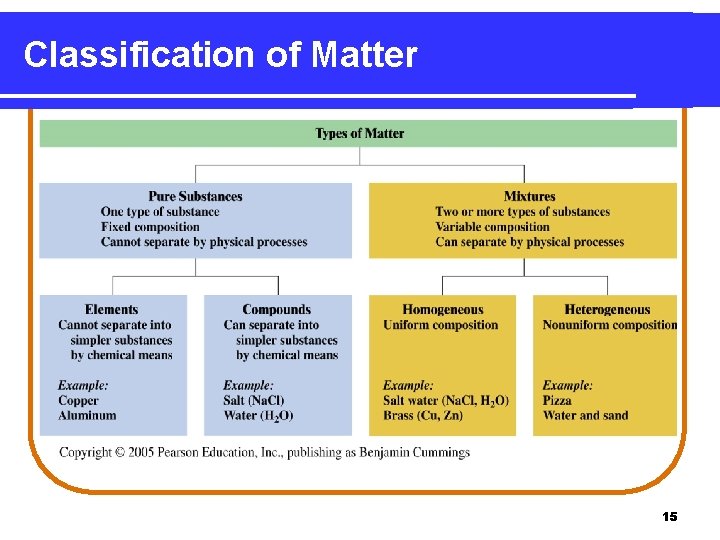
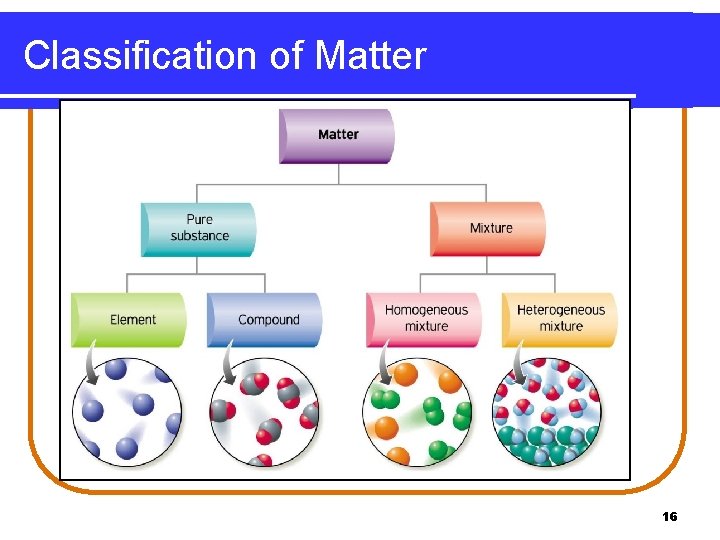
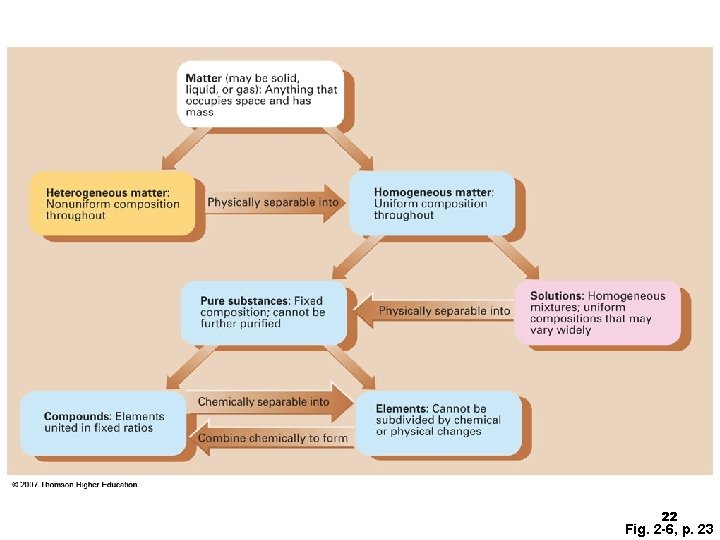
Matter 1. Types of Matter Pure substances (elements or compounds) b) Mixtures (homogeneous or heterogeneous) a) 2. States of Matter Solids, liquids, gases are the common states of matter. The fourth state, plasmas, occurs in flames, stars, and the outer atmosphere of Earth a) The kinds of matter described – elements, compounds, and mixtures– can be classified according to their composition and how they can be separated into other substances. 2

Pure Substances A pure substance is classified as • matter with a specific composition. • an element when composed of one type of atom. • a compound when composed of two or more elements combined in a definite ratio. 3

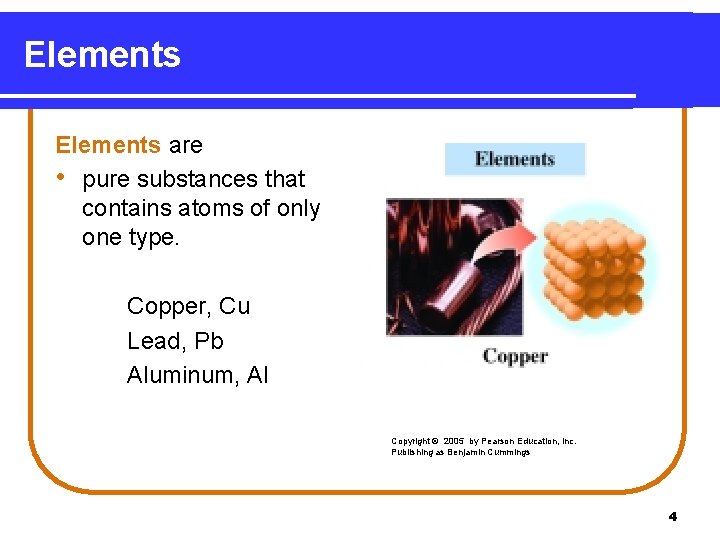
Elements are • pure substances that contains atoms of only one type. Copper, Cu Lead, Pb Aluminum, Al Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 4

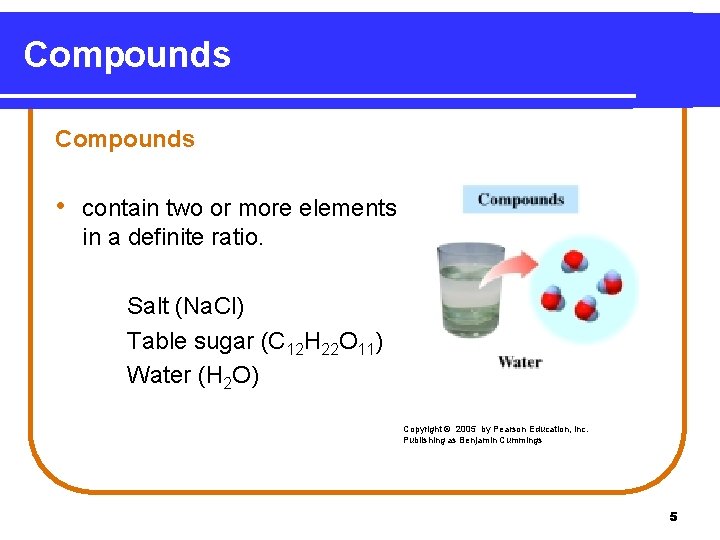
Compounds • contain two or more elements in a definite ratio. Salt (Na. Cl) Table sugar (C 12 H 22 O 11) Water (H 2 O) Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 5

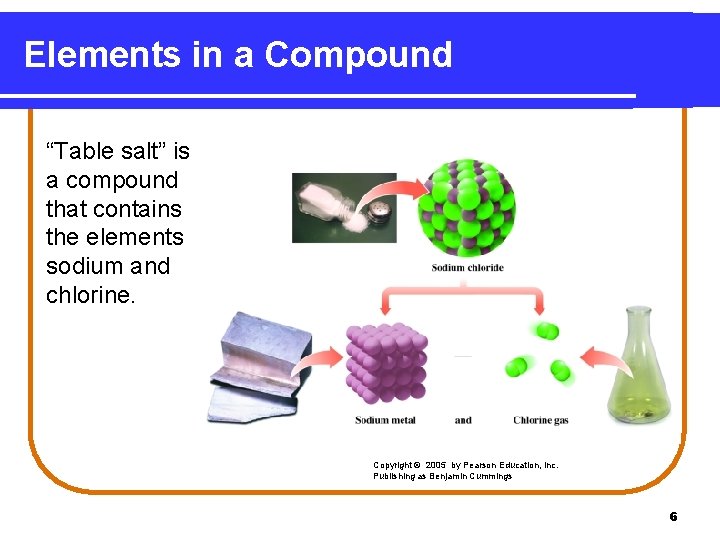
Elements in a Compound “Table salt” is a compound that contains the elements sodium and chlorine. Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 6

Mixtures A mixture is a type of matter that consists of • two or more substances that are physically mixed, not chemically combined. • two or more substances in different proportions. • substances that can be separated by physical methods. 7

Physical Separation of A Mixture Example: Pasta and water are separated with a strainer. 8

Homogeneous Mixtures In a homogeneous mixture, • the composition is uniform • throughout. the different parts of the mixture are not visible. Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings Three types: solutions, colloids, and suspensions 9

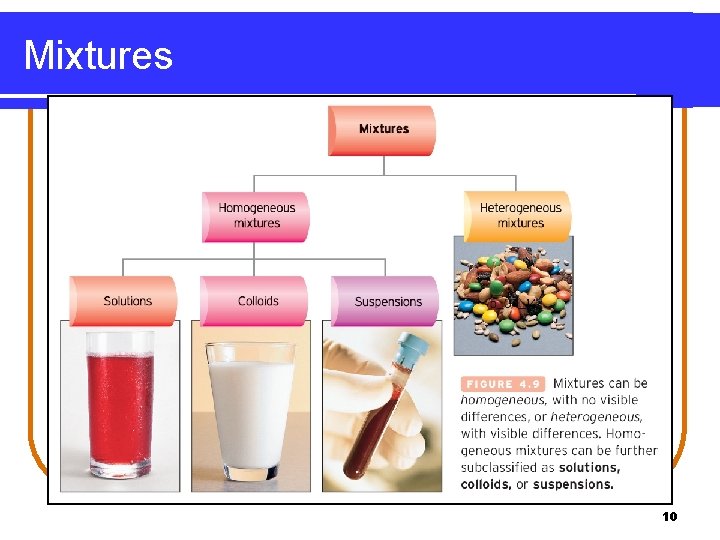
Mixtures 10

Homogeneous mixtures are uniform in composition and are referred to as solutions. Examples are: Order of Ice, trapping water molecules at the microscopic level. Also, clean air, freshly brewed ice tea, brass alloys, sugar, or table salt Solutions are homogenous mixtures which may be in the solid, liquid, or gaseous state. Ex. Mixing sugar in water, salt in water or freshly brewed ice tea 11

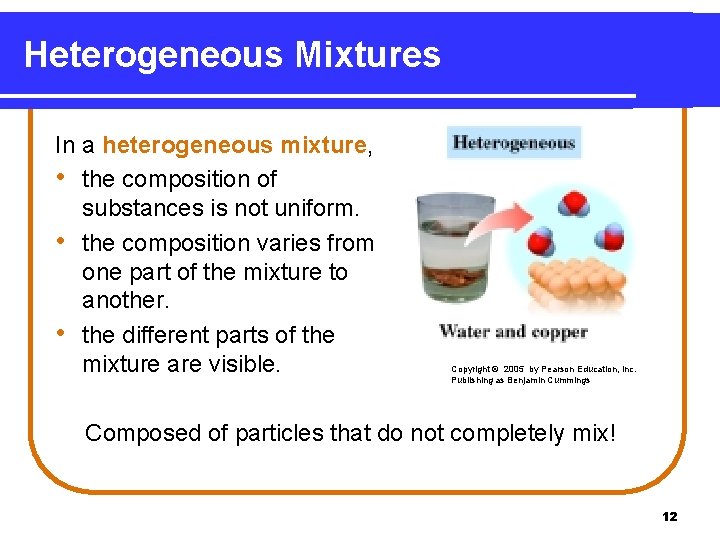
Heterogeneous Mixtures In a heterogeneous mixture, • the composition of substances is not uniform. • the composition varies from one part of the mixture to another. • the different parts of the mixture are visible. Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings Composed of particles that do not completely mix! 12

Heterogeneous mixtures are not uniform in composition and clearly visible, such as chocolate chip cookies or the different kinds of crystals in many rocks. 13

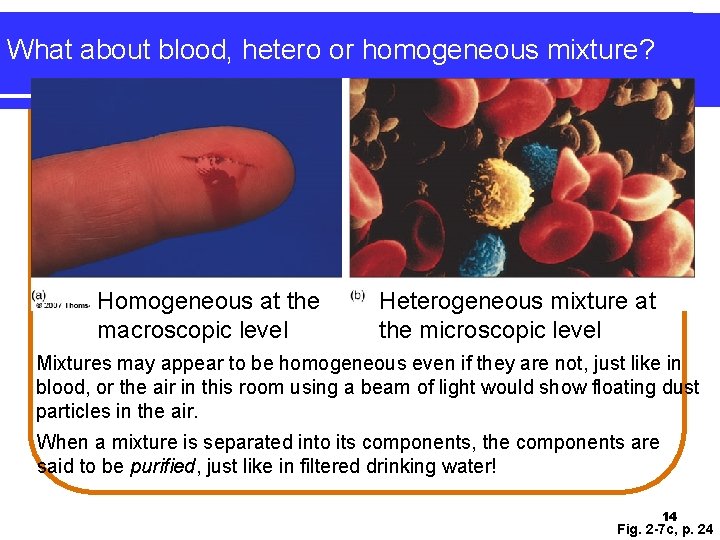
What about blood, hetero or homogeneous mixture? Homogeneous at the macroscopic level Heterogeneous mixture at the microscopic level Mixtures may appear to be homogeneous even if they are not, just like in blood, or the air in this room using a beam of light would show floating dust particles in the air. When a mixture is separated into its components, the components are said to be purified, just like in filtered drinking water! 14 Fig. 2 -7 c, p. 24

Classification of Matter 15

Classification of Matter 16

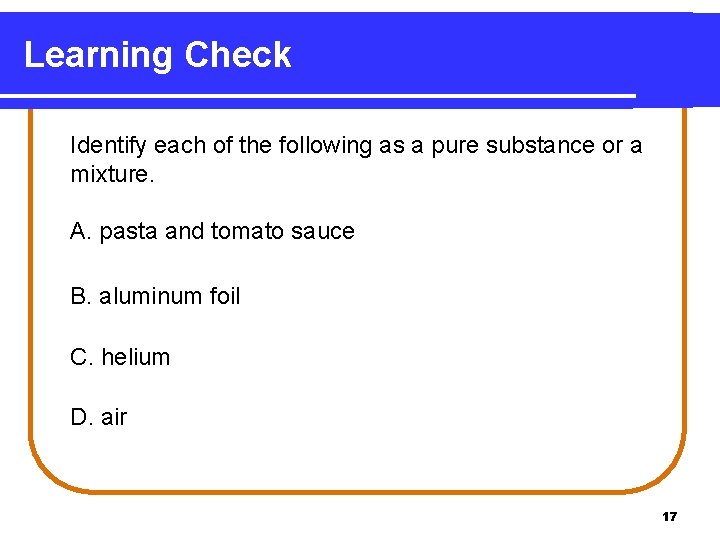
Learning Check Identify each of the following as a pure substance or a mixture. A. pasta and tomato sauce B. aluminum foil C. helium D. air 17

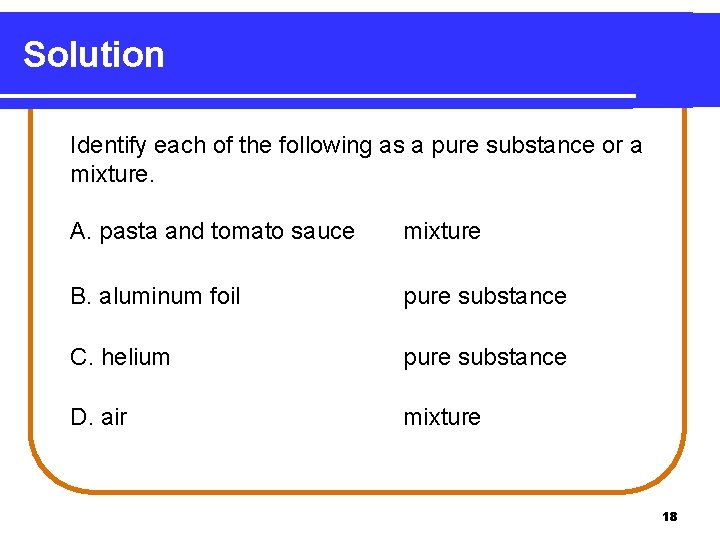
Solution Identify each of the following as a pure substance or a mixture. A. pasta and tomato sauce mixture B. aluminum foil pure substance C. helium pure substance D. air mixture 18

Learning Check Identify each of the following as a homogeneous or heterogeneous mixture: A. hot fudge sundae B. shampoo C. sugar water D. peach pie 19

Solution Identify each of the following as a homogeneous or heterogeneous mixture: A. hot fudge sundae heterogeneous mixture B. shampoo homogeneous mixture C. sugar water homogeneous mixture D. peach pie heterogeneous mixture 20

Experimentally, pure substances are classified into 2 categories: 1. Chemical compounds, which can be broken down into simpler substances called elements. 2. Elements, which cannot be broken down into any smaller particle still recognizable as that element. 21

22 Fig. 2 -6, p. 23