SOLUTIONS A homogeneous mixture in which the components







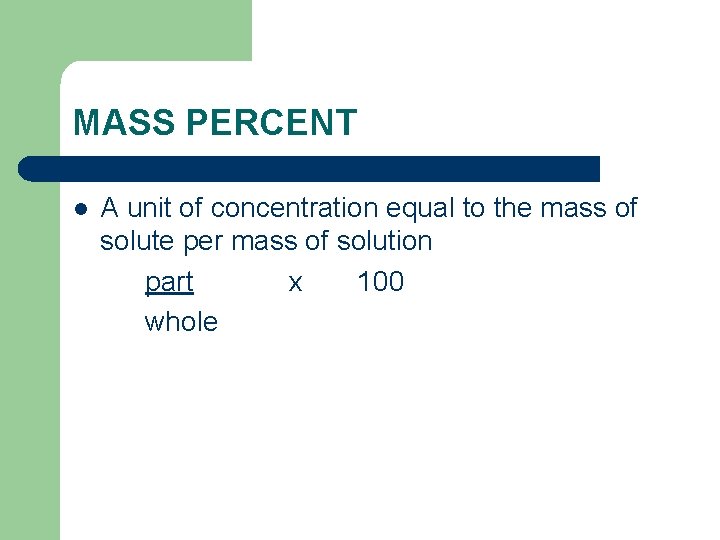

- Slides: 14

SOLUTIONS A homogeneous mixture in which the components are uniformly intermingled

Terms Solvent – The substance present in the largest amount in a solution. The substance that does the dissolving. Solute – The other substance or substances in a solution. The substance that is dissolved.

ELECTROLYTES l l l Substances that break up in water to produce ions. These ions can conduct electric current Examples: Acids, Bases and Salts (ionic compounds)

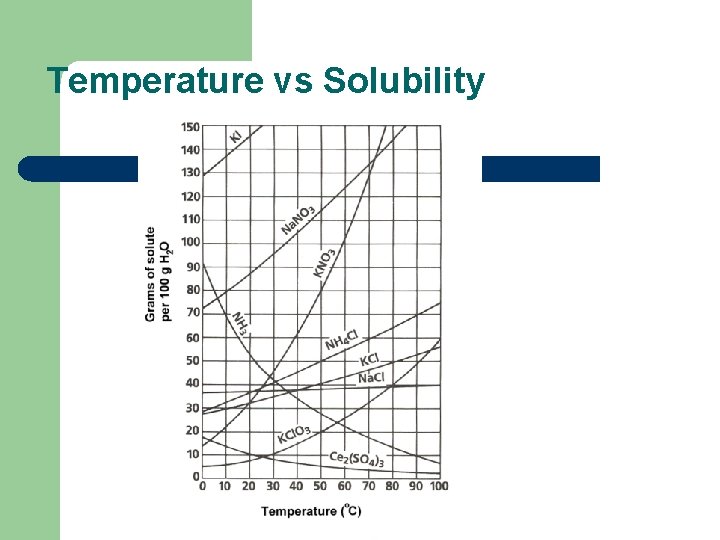
SOLUBILITY l l Is the amount of a substance that dissolves in 100 g of water at a given temperature to produce a saturated solution “Like dissolves Like” – – Polar molecules dissolve polar molecules Nonpolar molecules dissolve nonpolar molecules

SOLUBILITY RULES l l l All common salts of Group I elements and ammonium are soluble All common acetates and nitrates are soluble All binary compounds of Group 7 (other than F) with metals are soluble except those of silver, mercury I and lead All sulfates are soluble except those of barium, strontium, calcium, silver, mercury I and lead Except for those in Rule 1, carbonates, hydroxides, sulfides and phosphates are insoluble

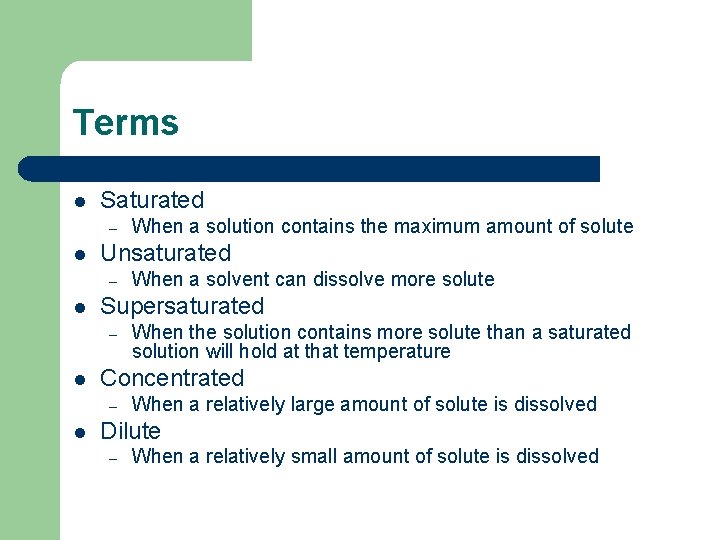
Terms l Saturated – l Unsaturated – l When the solution contains more solute than a saturated solution will hold at that temperature Concentrated – l When a solvent can dissolve more solute Supersaturated – l When a solution contains the maximum amount of solute When a relatively large amount of solute is dissolved Dilute – When a relatively small amount of solute is dissolved

Factors Affecting the Rate of Dissolution l Surface Area l Stirring l Temperature

Temperature vs Solubility

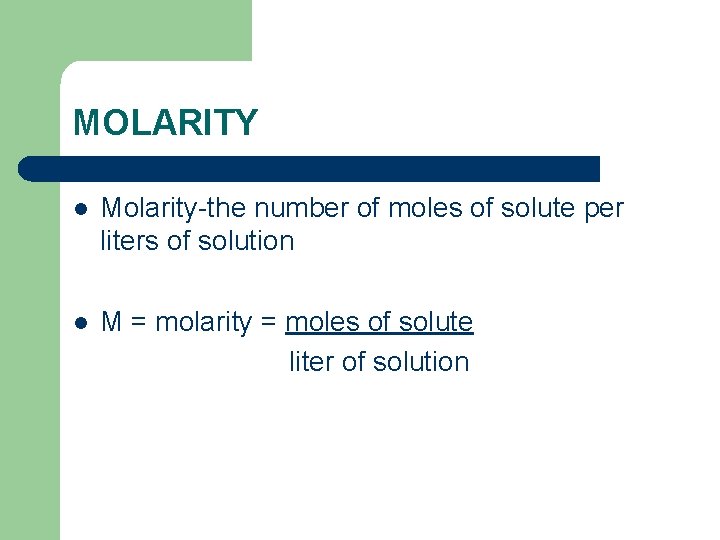
MOLARITY l Molarity-the number of moles of solute per liters of solution l M = molarity = moles of solute liter of solution

l Calculate the molarity of a solution prepared by dissolving 11. 5 g of Na. OH in enough water to make a 1. 50 L solution.

l Calculate the molarity of a solution prepared by dissolving 1. 56 g of HCl into enough water to make 26. 8 ml of solution.

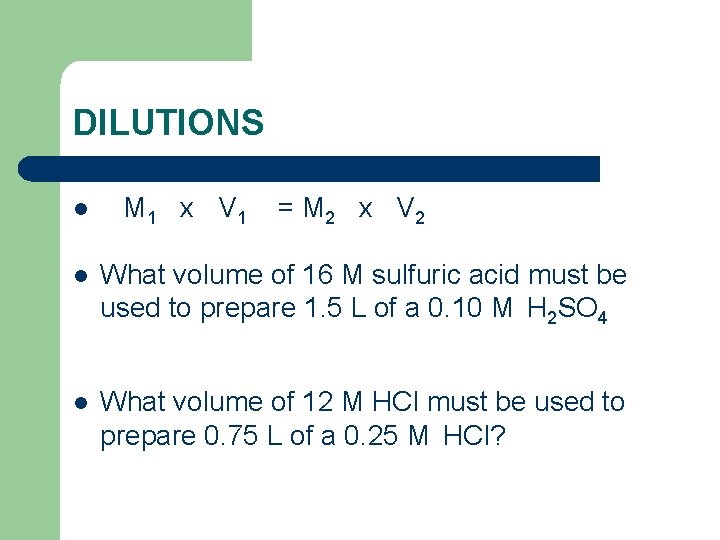
DILUTIONS l M 1 x V 1 = M 2 x V 2 l What volume of 16 M sulfuric acid must be used to prepare 1. 5 L of a 0. 10 M H 2 SO 4 l What volume of 12 M HCl must be used to prepare 0. 75 L of a 0. 25 M HCl?
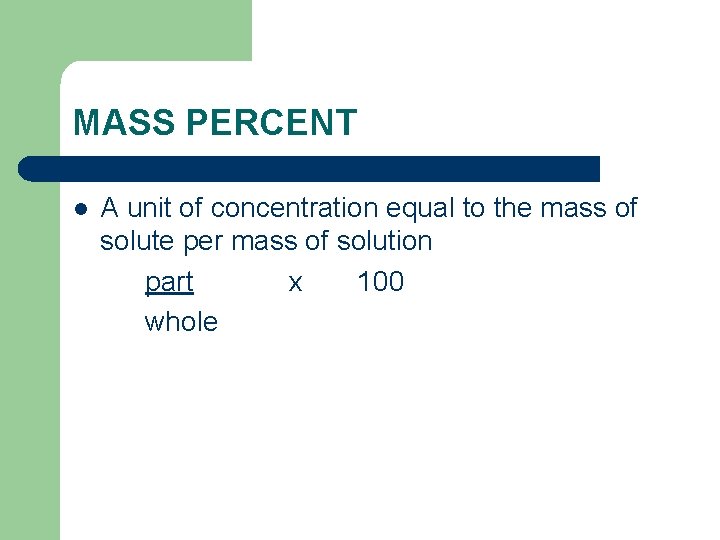
MASS PERCENT l A unit of concentration equal to the mass of solute per mass of solution part x 100 whole

l A solution is prepared by mixing 1. 00 g of ethanol with 100. 0 g of water. Calculate the mass percent of ethanol in this solution. l A 135 g sample of seawater is evaporated to dryness, leaving 4. 73 g of salt. Calculate the mass percent of salt in the saltwater.