Solutions n Solution a homogeneous mixture of pure












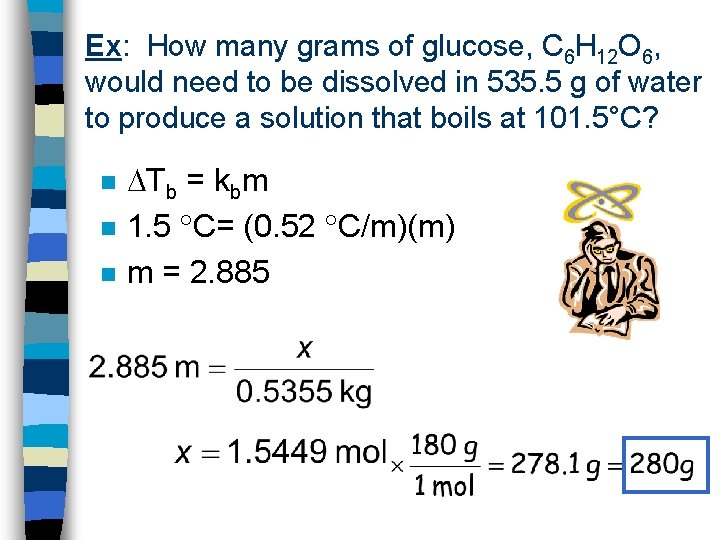





- Slides: 101


Solutions n Solution – a homogeneous mixture of pure substances (occur in all phases, but we will focus on aqueous solutions) n The SOLVENT is the medium in which the SOLUTES are dissolved. (The solvent is usually the most abundant substance. ) – Example: • Solution: Salt Water • Solute: Salt • Solvent: Water

Concentration of Solution n Concentration refers to the amount of solute dissolved in a solution. Ways of measuring concentration…

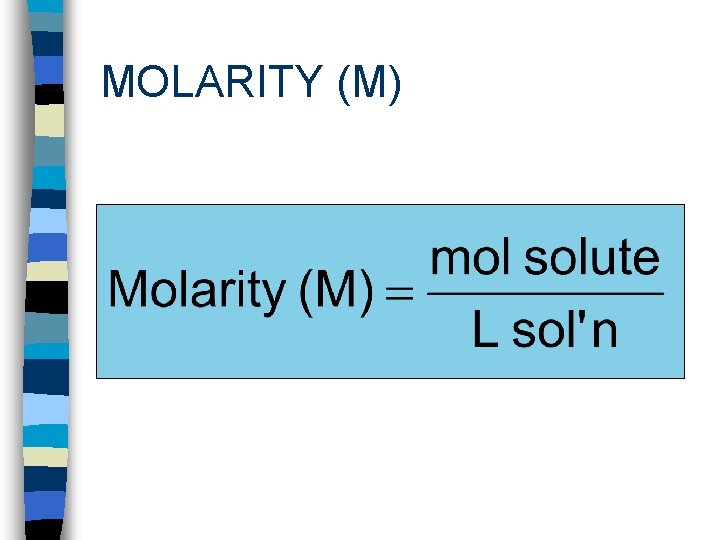
MOLARITY (M)

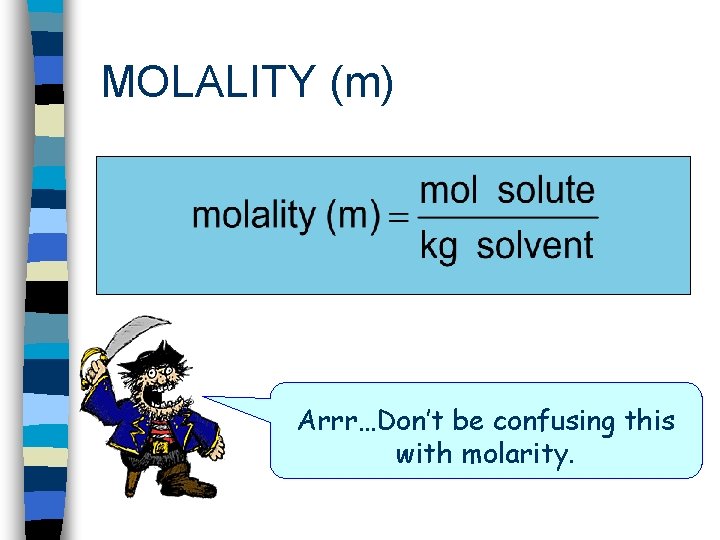
MOLALITY (m) Arrr…Don’t be confusing this with molarity.

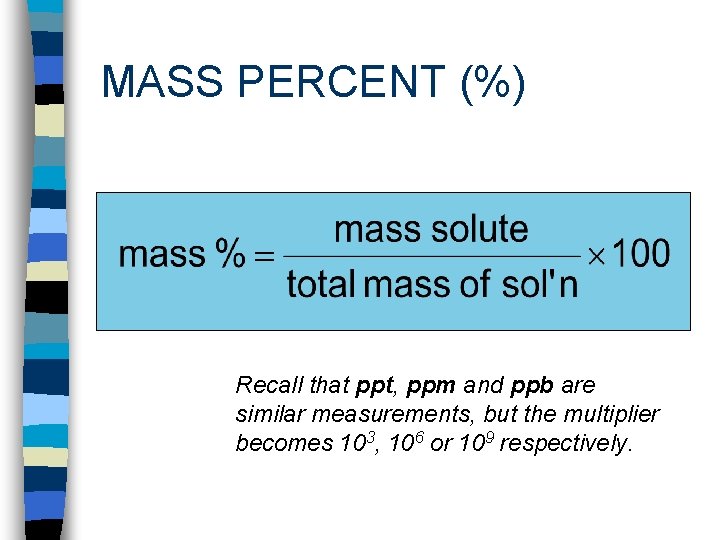
MASS PERCENT (%) Recall that ppt, ppm and ppb are similar measurements, but the multiplier becomes 103, 106 or 109 respectively.

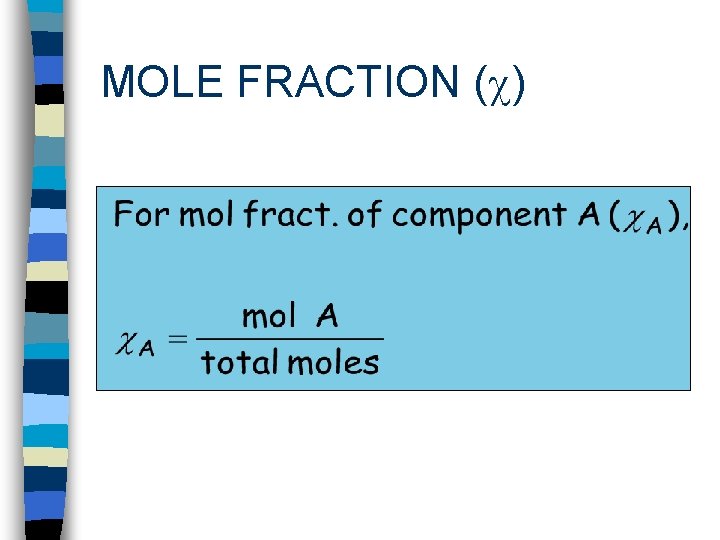
MOLE FRACTION ( )

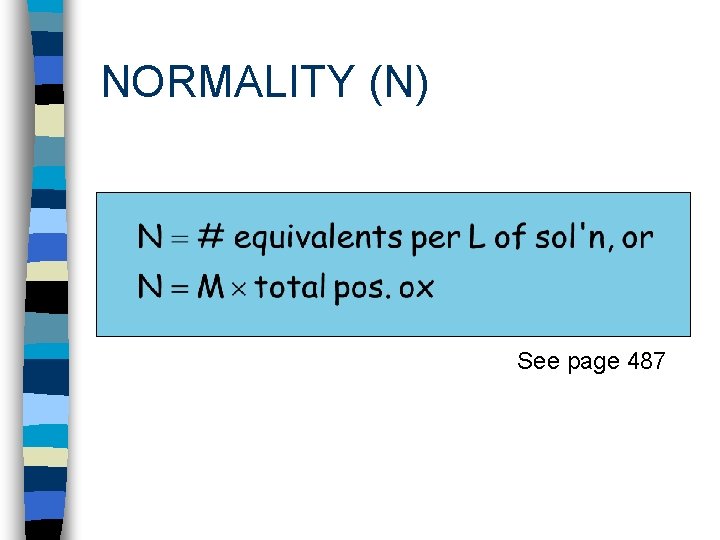
NORMALITY (N) See page 487

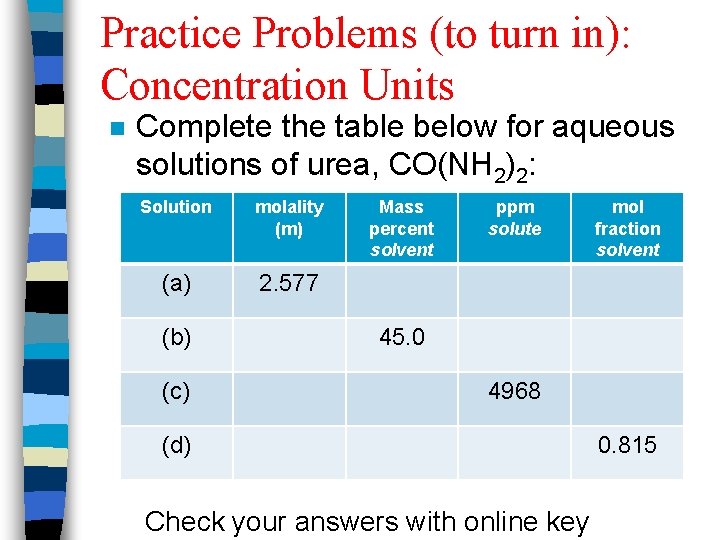
Practice Problems (to turn in): Concentration Units n Complete the table below for aqueous solutions of urea, CO(NH 2)2: Solution molality (m) (a) 2. 577 (b) (c) Mass percent solvent ppm solute mol fraction solvent 45. 0 4968 (d) Check your answers with online key 0. 815

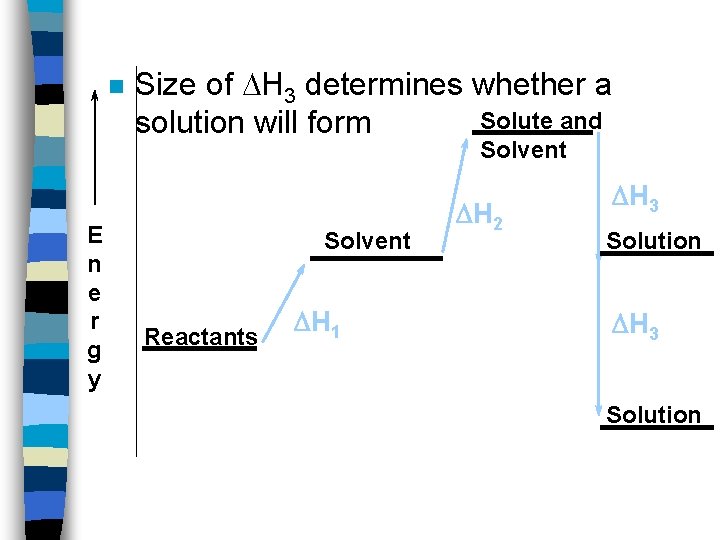
Energy of Making Solutions n n Heat of solution ( Hsoln ) is the energy change for making a solution. Most easily understood if broken into steps. 1. Break apart solvent 2. Break apart solute 3. Mixing solvent and solute

1. Break apart Solvent n Have to overcome attractive forces. H 1 >0 2. Break apart Solute. n Have to overcome attractive forces. H 2 >0

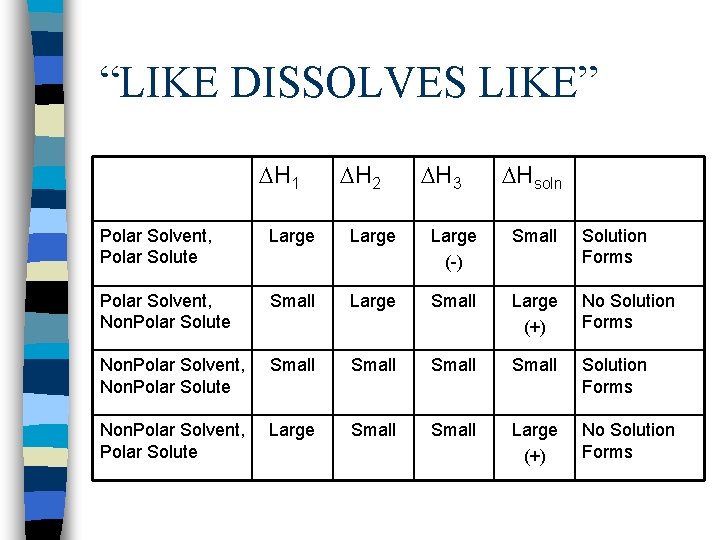
3. Mixing solvent and solute n n H 3 depends on what you are mixing. Molecules can attract each other - H 3 is large and negative. Molecules can’t attract - H 3 is small and negative. This explains the rule “like dissolves like”

n Size of H 3 determines whether a Solute and solution will form Solvent E n e r g y Solvent Reactants H 1 H 2 H 3 Solution

Types of Solvent and solutes n n If Hsoln is small and positive, a solution will still form because of entropy. There are many more ways for them to become mixed than there is for them to stay separate.

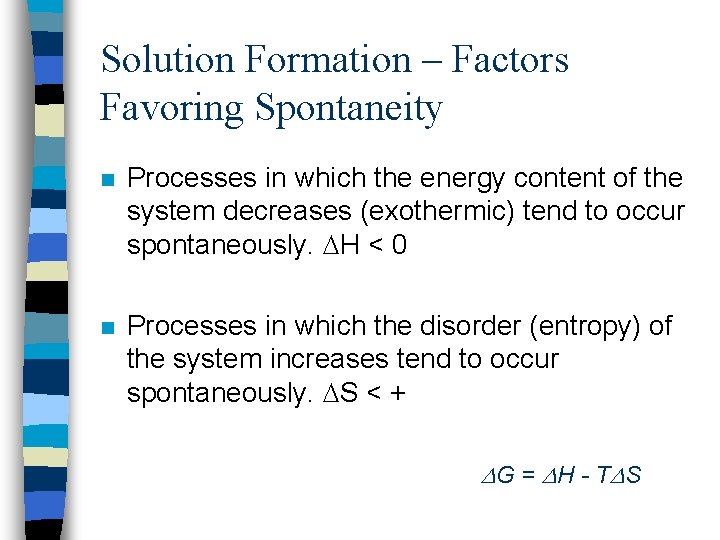
Solution Formation – Factors Favoring Spontaneity n Processes in which the energy content of the system decreases (exothermic) tend to occur spontaneously. H < 0 n Processes in which the disorder (entropy) of the system increases tend to occur spontaneously. S < + G = H - T S

“LIKE DISSOLVES LIKE” H 1 H 2 H 3 Hsoln Polar Solvent, Polar Solute Large (-) Small Solution Forms Polar Solvent, Non. Polar Solute Small Large (+) No Solution Forms Non. Polar Solvent, Non. Polar Solute Small Solution Forms Non. Polar Solvent, Polar Solute Large Small Large (+) No Solution Forms

It seems the extent to which one substance dissolves in another depends on the nature of both the solute and the solvent. (“like dissolves like”). It also depends on temperature and at least for gases, on pressure.

As a rule, network covalent solids, such as graphite and quartz (Si. O 2), do not dissolve in any solvent. Nor do metals, technically speaking “dissolve. ” Ionic and molecular compounds, on the other hand will dissolve in certain solvents.

Structure and Solubility n Water soluble molecules must have dipole moments -polar bonds. n To be soluble in non polar solvents the molecules must be non polar.

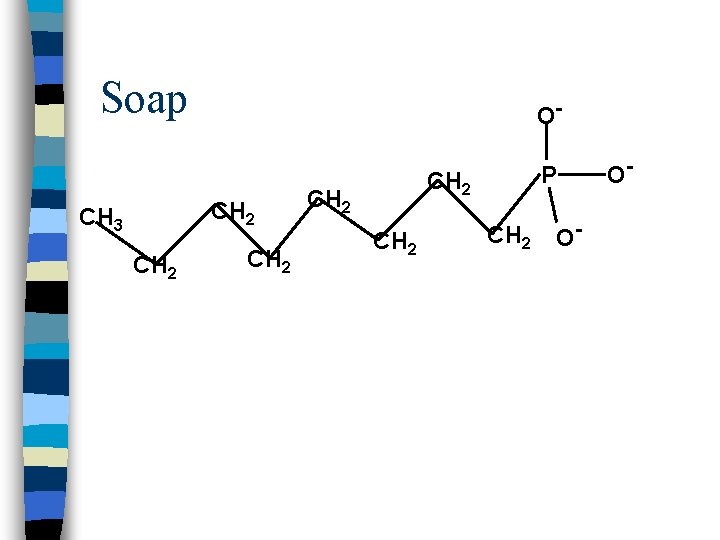
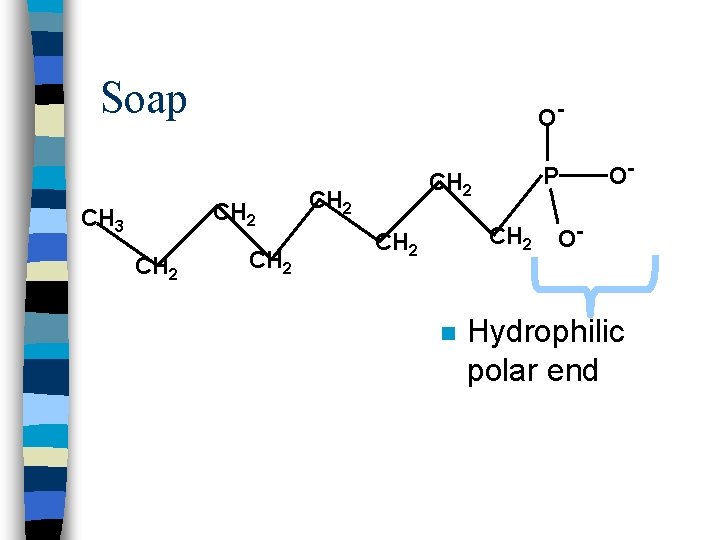
Soap O- CH 2 CH 3 CH 2 P CH 2 O- O-

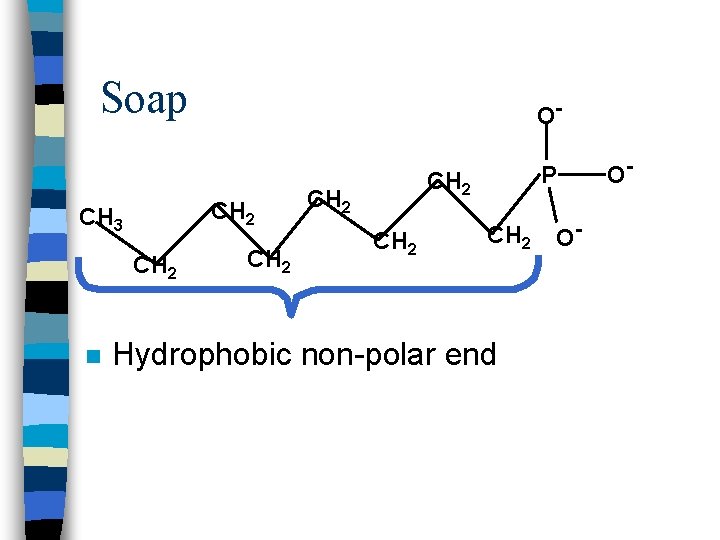
Soap CH 2 CH 3 CH 2 n O- CH 2 P CH 2 Hydrophobic non-polar end O- O-

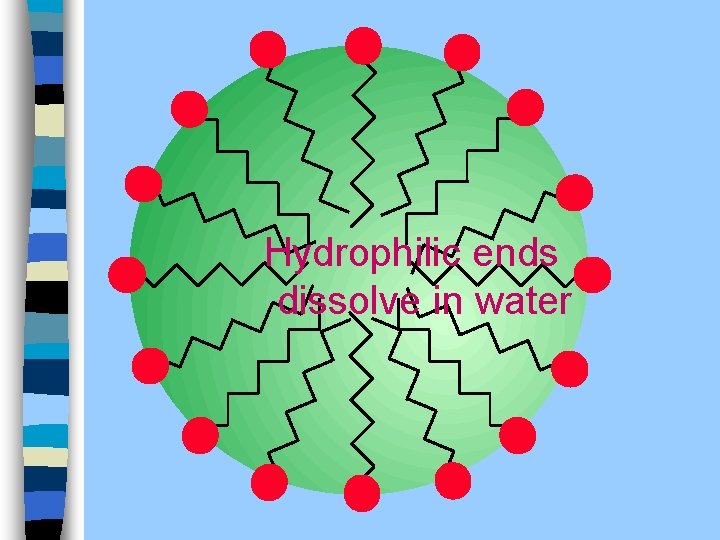
Soap O- CH 2 CH 3 CH 2 P CH 2 n O- O- Hydrophilic polar end

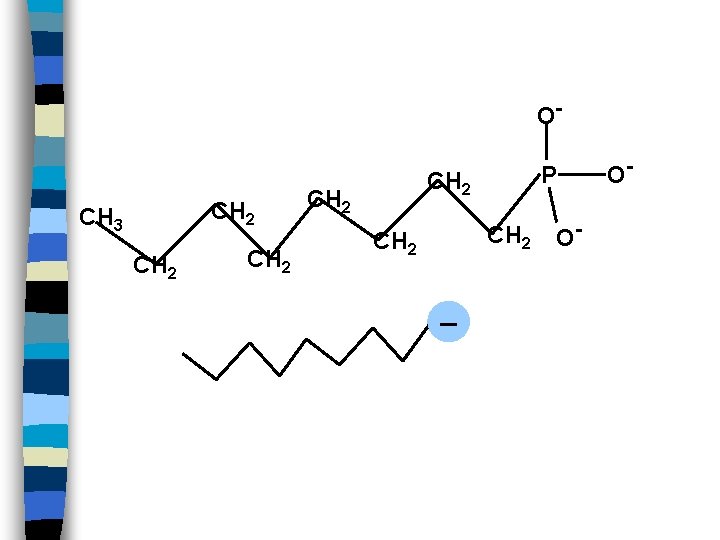
O- CH 2 CH 3 CH 2 P CH 2 _ O- O-

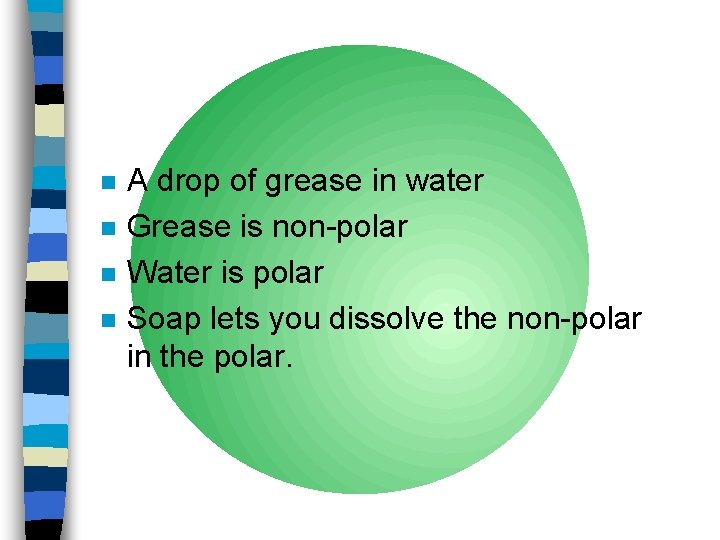
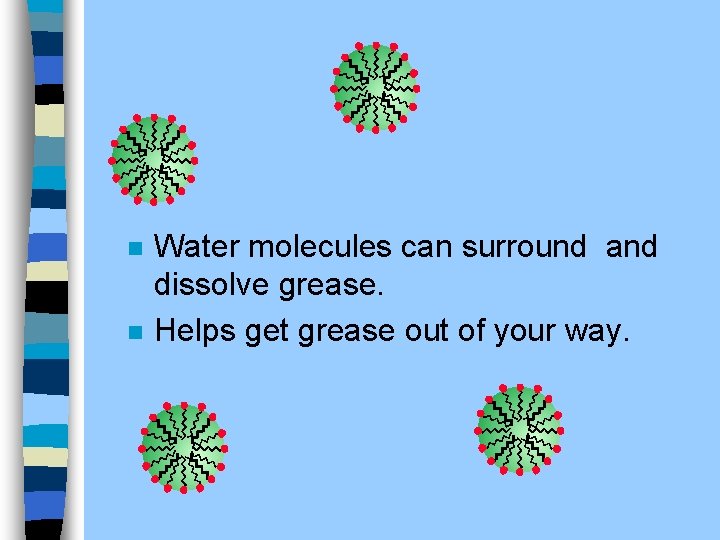
n n A drop of grease in water Grease is non-polar Water is polar Soap lets you dissolve the non-polar in the polar.

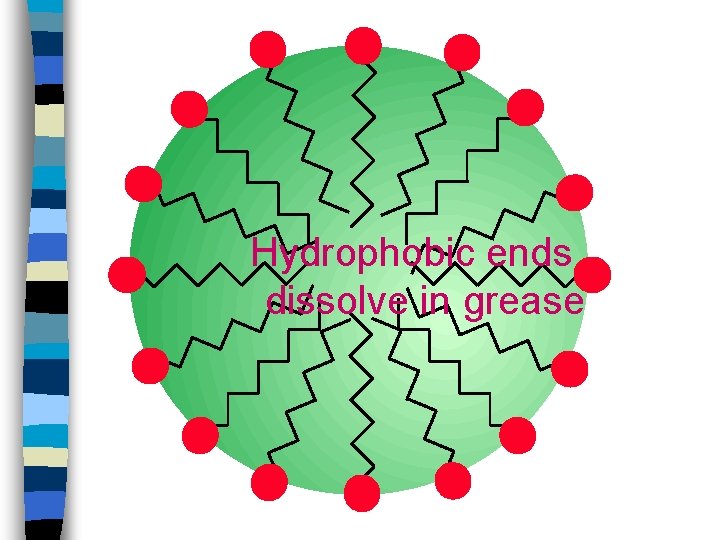
Hydrophobic ends dissolve in grease

Hydrophilic ends dissolve in water

n n Water molecules can surround and dissolve grease. Helps get grease out of your way.

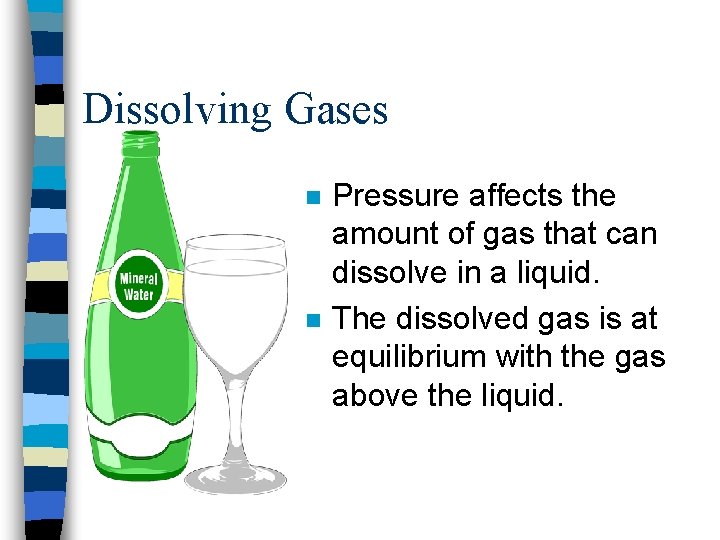
Pressure effects n Changing the pressure doesn’t affect the amount of solid or liquid that dissolves – They are incompressible. n Pressure does affect solubility of gases.

Dissolving Gases n n Pressure affects the amount of gas that can dissolve in a liquid. The dissolved gas is at equilibrium with the gas above the liquid.

n n The gas is at equilibrium with the dissolved gas in this solution. The equilibrium is dynamic.

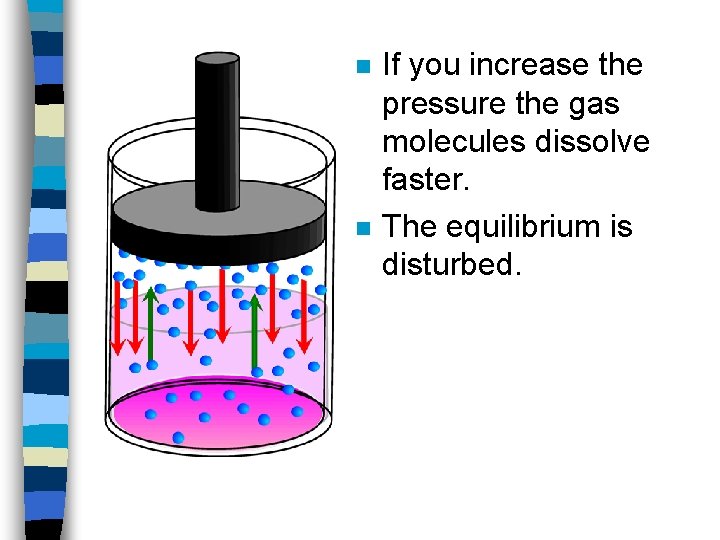
n n If you increase the pressure the gas molecules dissolve faster. The equilibrium is disturbed.

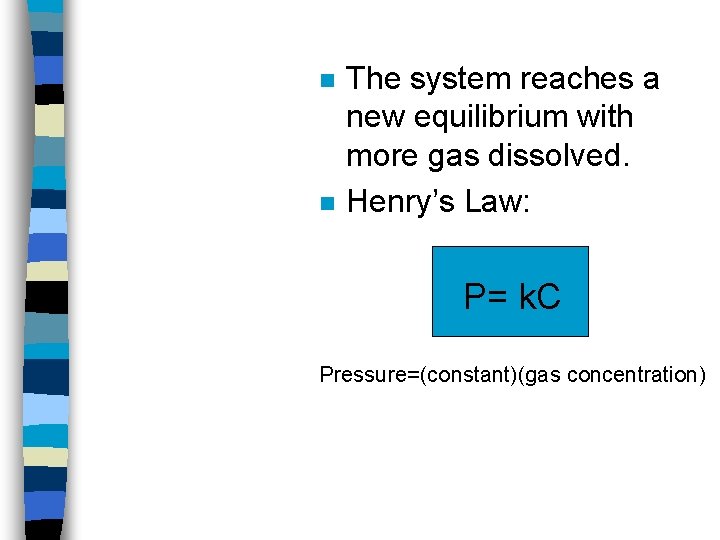
n n The system reaches a new equilibrium with more gas dissolved. Henry’s Law: P= k. C Pressure=(constant)(gas concentration)

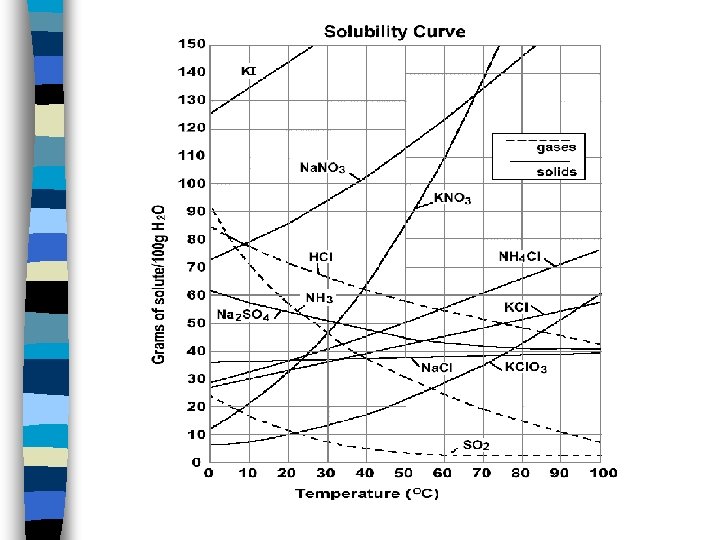
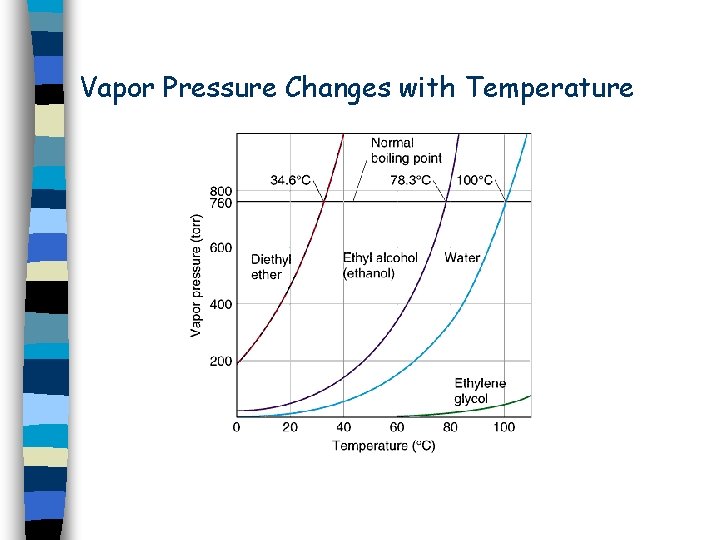
Temperature Effects n n n Increased temperature increases the rate at which a solid dissolves. We can’t predict whether it will increase the amount of solid that dissolves. We must read it from a graph of experimental data.

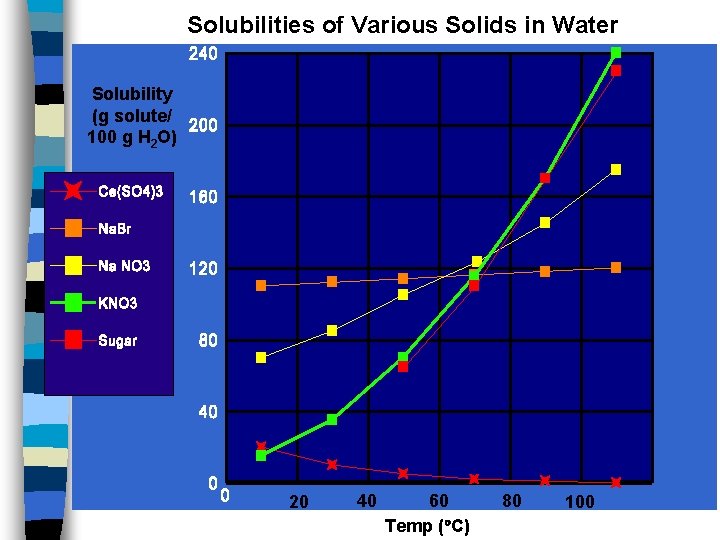
Solubilities of Various Solids in Water Solubility (g solute/ 100 g H 2 O) 20 40 60 Temp ( C) 80 100

Gases are predictable n n n As temperature increases, solubility decreases. Gas molecules can move fast enough to escape. Thermal pollution.


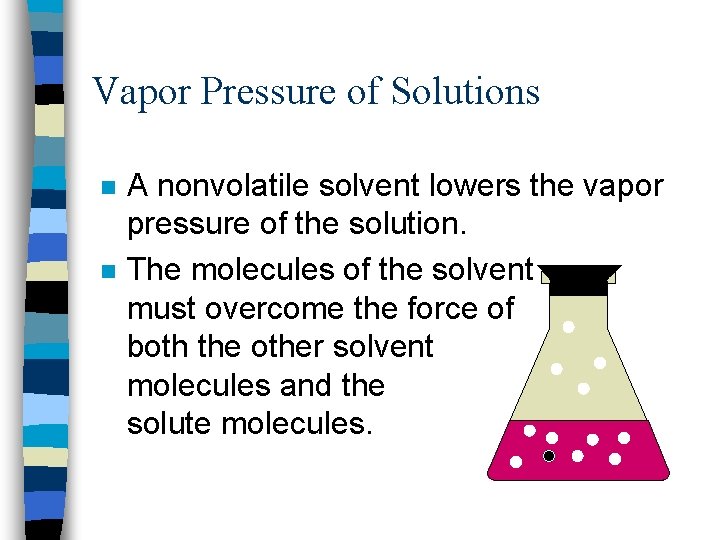
Vapor Pressure of Solutions n n A nonvolatile solvent lowers the vapor pressure of the solution. The molecules of the solvent must overcome the force of both the other solvent molecules and the solute molecules.

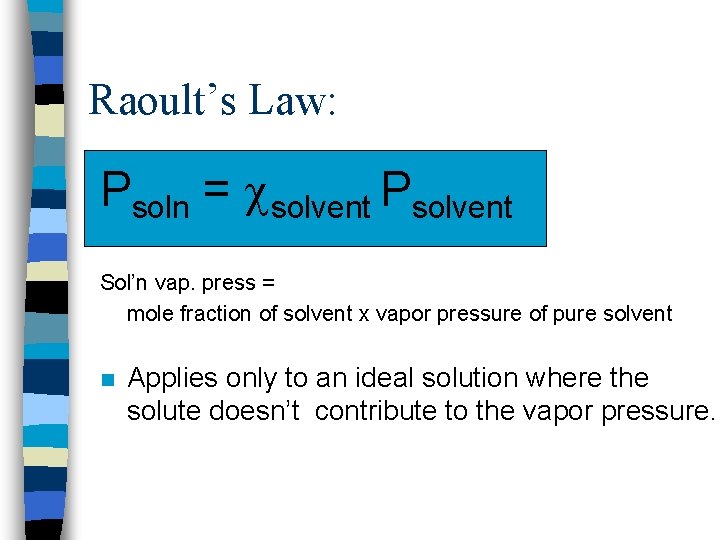
Raoult’s Law: Psoln = solvent Psolvent Sol’n vap. press = mole fraction of solvent x vapor pressure of pure solvent n Applies only to an ideal solution where the solute doesn’t contribute to the vapor pressure.

To determine whether a sol’n is IDEAL… n Liquid-liquid solutions where both are volatile. n Modify Raoult’s Law to Ptotal = PA + PB = APA 0 + BPB 0 • Ptotal = vapor pressure of mixture • PA 0= vapor pressure of pure A n n If this equation works then the solution is ideal. Solvent and solute are alike.

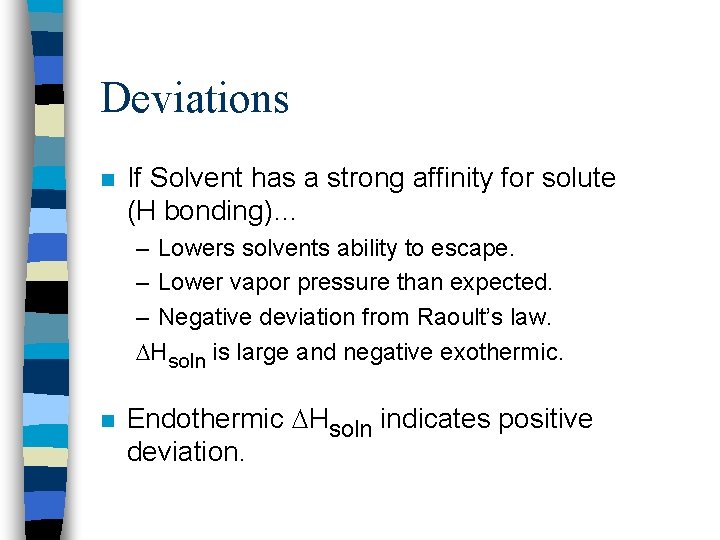
Deviations n If Solvent has a strong affinity for solute (H bonding)… – Lowers solvents ability to escape. – Lower vapor pressure than expected. – Negative deviation from Raoult’s law. Hsoln is large and negative exothermic. n Endothermic Hsoln indicates positive deviation.

Colligative Properties of Solutions Colligative properties = physical properties of solutions that depend on the # of particles dissolved, not the kind of particle.

Colligative Properties n n Lowering vapor pressure Raising boiling point Lowering freezing point Generating an osmotic pressure

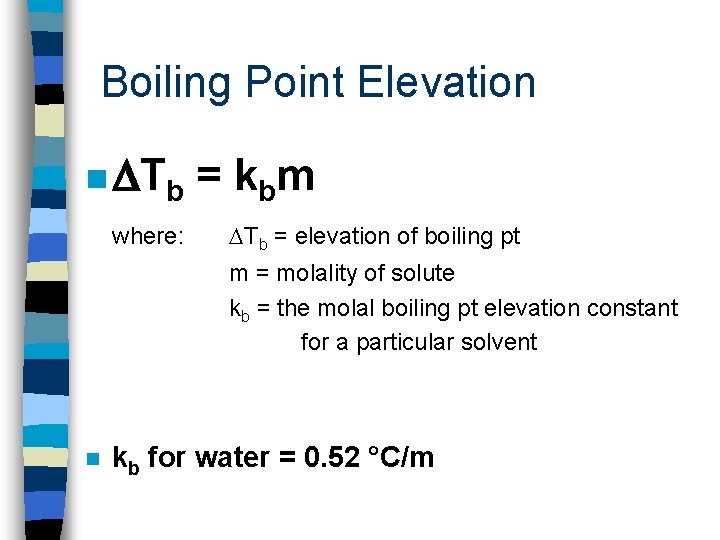
Boiling Point Elevation n a solution that contains a nonvolatile solute has a higher boiling pt than the pure solvent; the boiling pt elevation is proportional to the # of moles of solute dissolved in a given mass of solvent.

n Ok… but WHY? ? ?

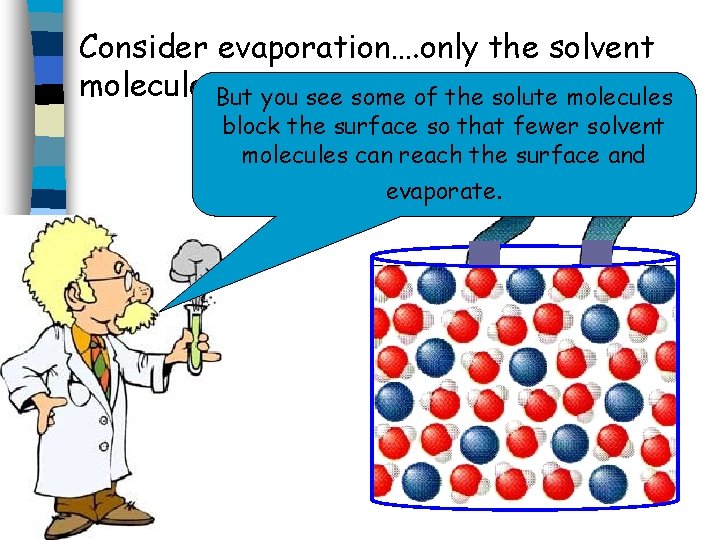
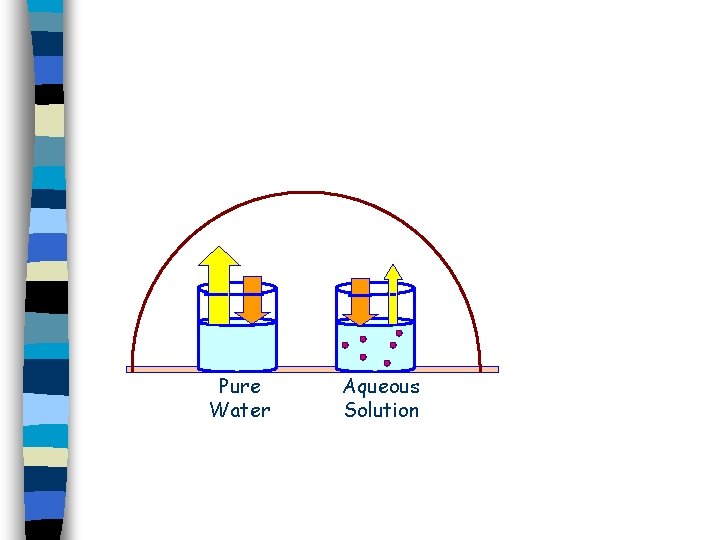
Consider evaporation…. only the solvent molecules. But evaporate. you see some of the solute molecules block the surface so that fewer solvent molecules can reach the surface and evaporate.

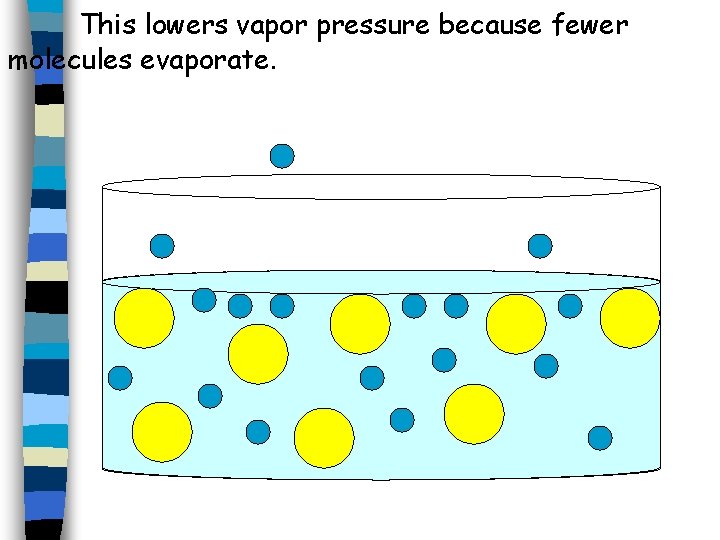
This lowers vapor pressure because fewer molecules evaporate.

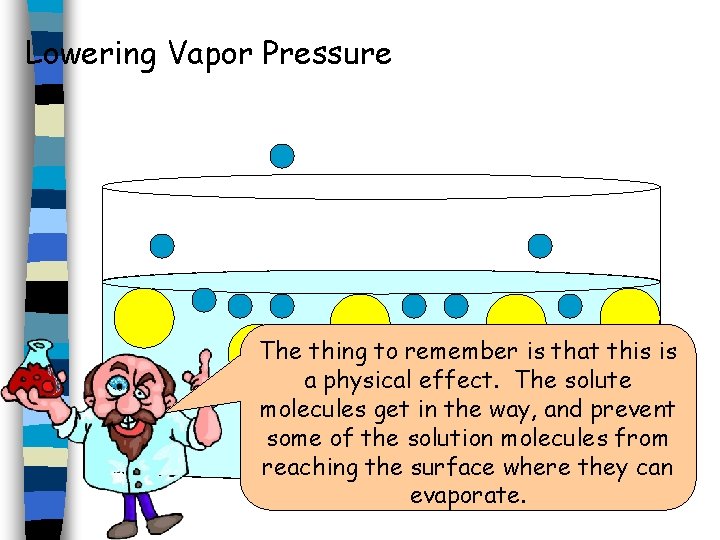
Lowering Vapor Pressure The thing to remember is that this is a physical effect. The solute molecules get in the way, and prevent some of the solution molecules from reaching the surface where they can evaporate.

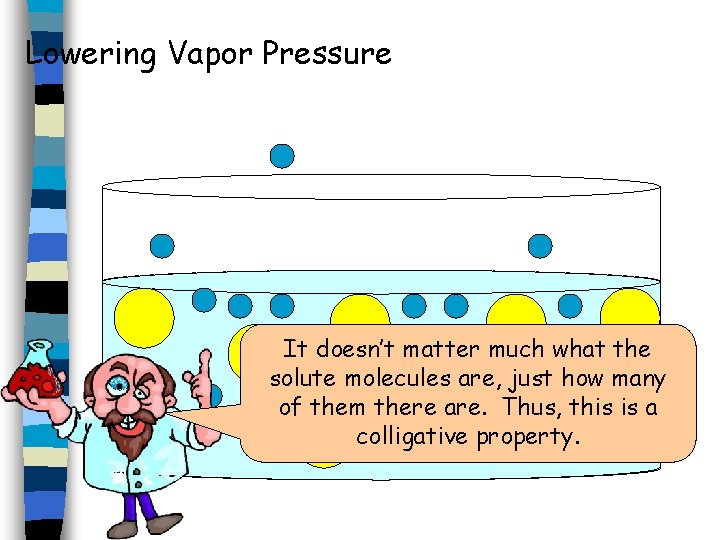
Lowering Vapor Pressure It doesn’t matter much what the solute molecules are, just how many of them there are. Thus, this is a colligative property.

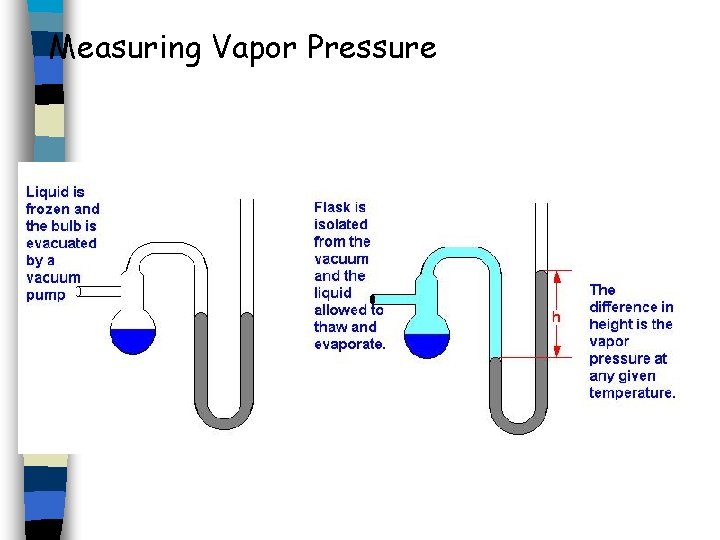
Measuring Vapor Pressure

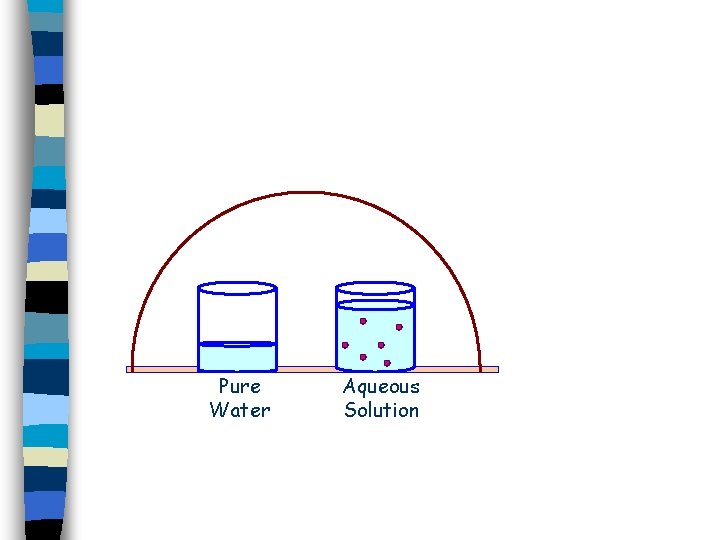
Pure Water Aqueous Solution

Pure Water Aqueous Solution

Pure Water Aqueous Solution

Pure Water Aqueous Solution

Pure Water Aqueous Solution

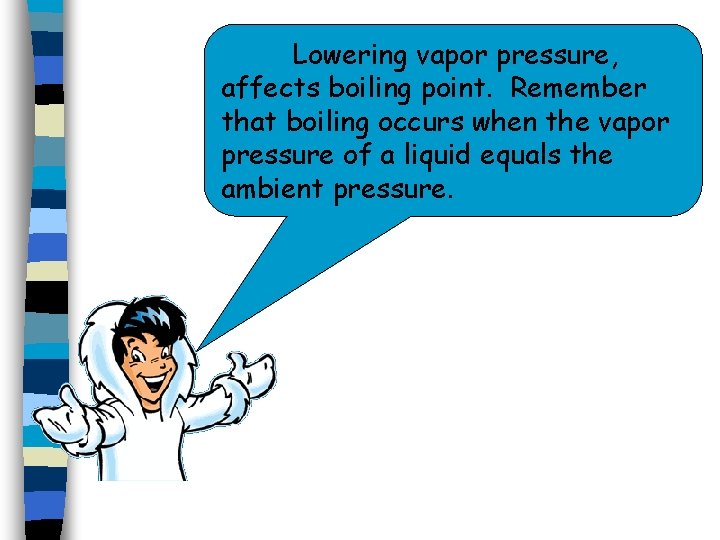
Lowering vapor pressure, affects boiling point. Remember that boiling occurs when the vapor pressure of a liquid equals the ambient pressure.

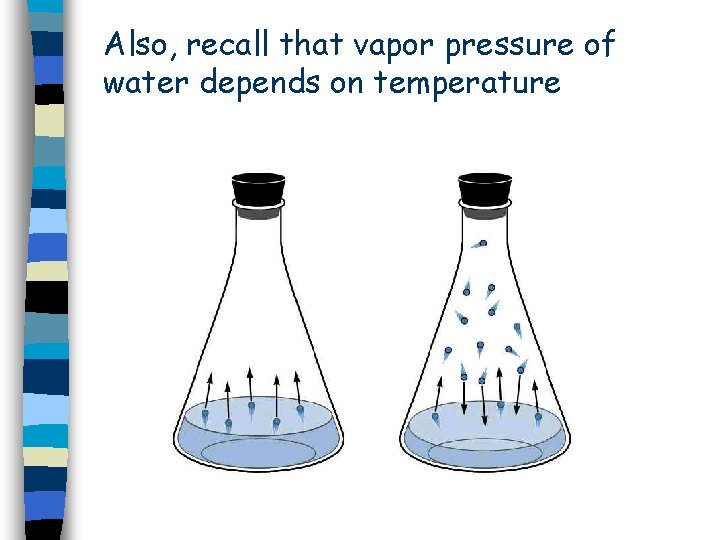
Also, recall that vapor pressure of water depends on temperature

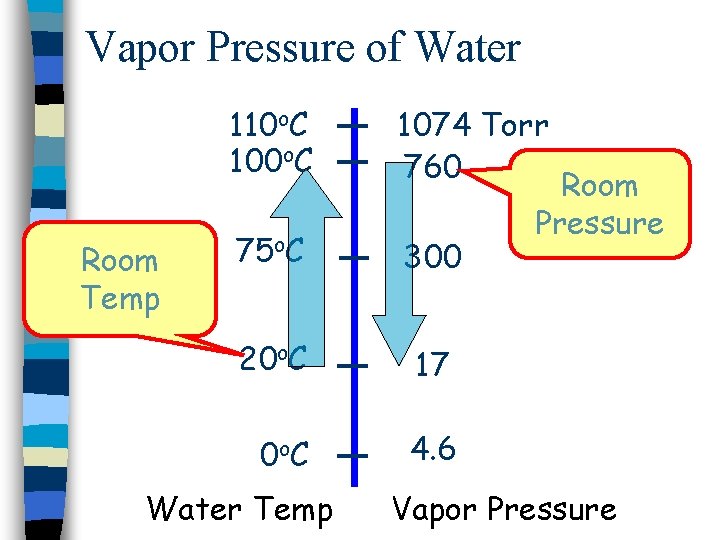
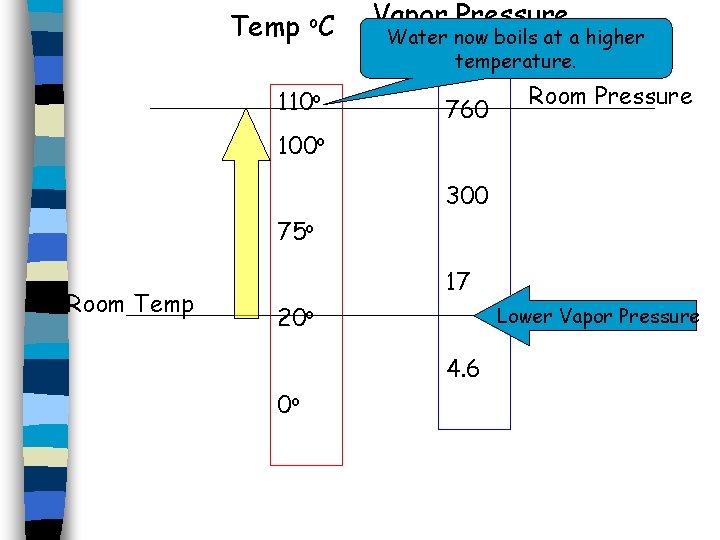
Vapor Pressure of Water Room Temp 110 o. C 1074 Torr 760 75 o. C 300 20 o. C 17 0 o C 4. 6 Water Temp Room Pressure Vapor Pressure

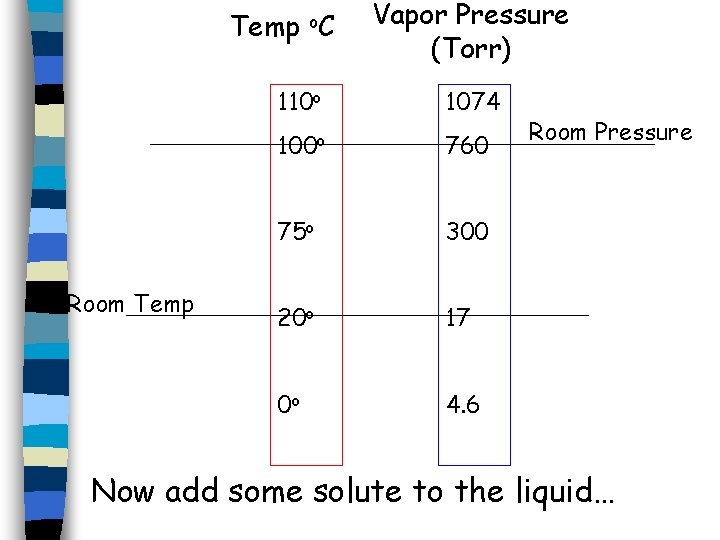
Temp o. C Room Temp Vapor Pressure (Torr) 110 o 1074 100 o 760 75 o 300 20 o 17 0 o 4. 6 Room Pressure Now add some solute to the liquid…

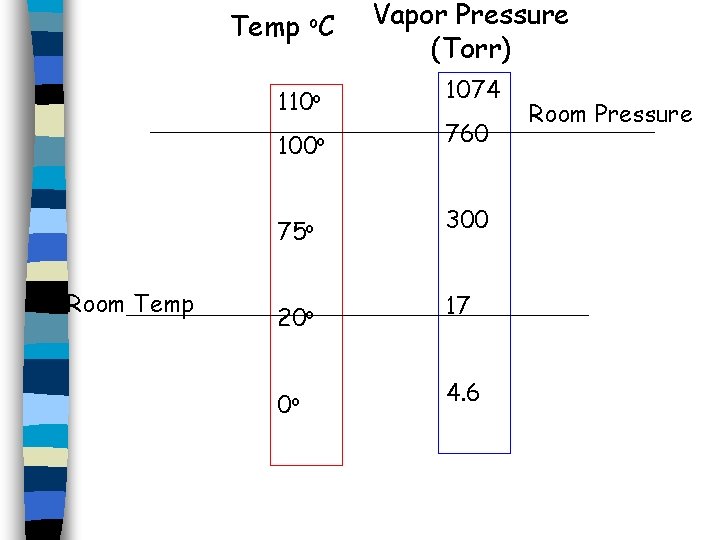
Temp o. C 110 o Room Temp Vapor Pressure (Torr) 1074 100 o 760 75 o 300 20 o 17 0 o 4. 6 Room Pressure

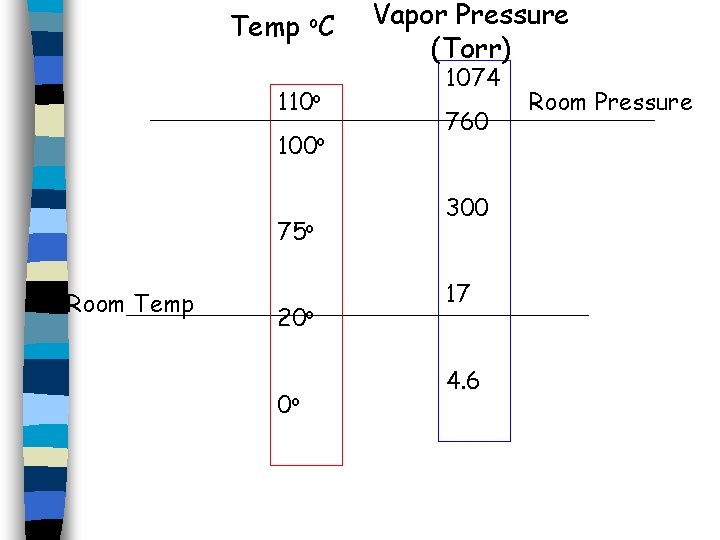
Temp o. C 110 o 100 o 75 o Room Temp 20 o 0 o Vapor Pressure (Torr) 1074 760 300 17 4. 6 Room Pressure

Temp o. C Vapor Pressure Water now boils at a higher (Torr) temperature. 1074 110 o 760 Room Pressure 100 o 300 75 o Room Temp 17 20 o Lower Vapor Pressure 4. 6 0 o

Vapor Pressure Changes with Temperature

Boiling Point Elevation n Tb where: = k bm Tb = elevation of boiling pt m = molality of solute kb = the molal boiling pt elevation constant for a particular solvent n kb for water = 0. 52 °C/m

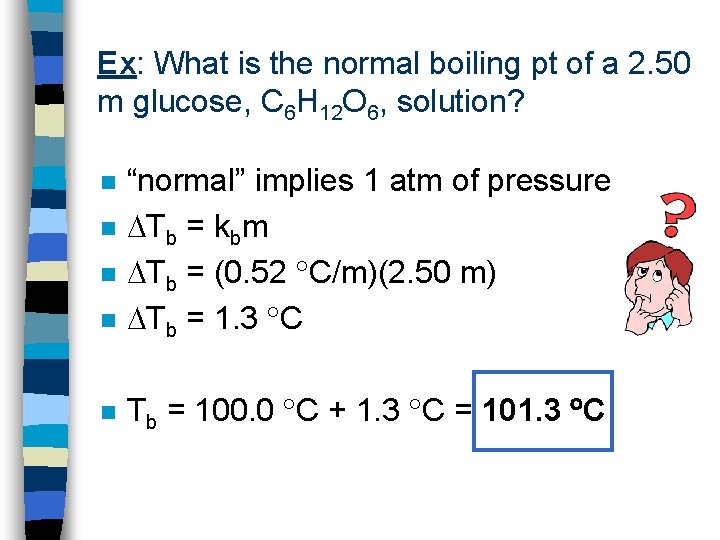
Ex: What is the normal boiling pt of a 2. 50 m glucose, C 6 H 12 O 6, solution? n “normal” implies 1 atm of pressure Tb = kbm Tb = (0. 52 C/m)(2. 50 m) Tb = 1. 3 C n Tb = 100. 0 C + 1. 3 C = 101. 3 C n n n
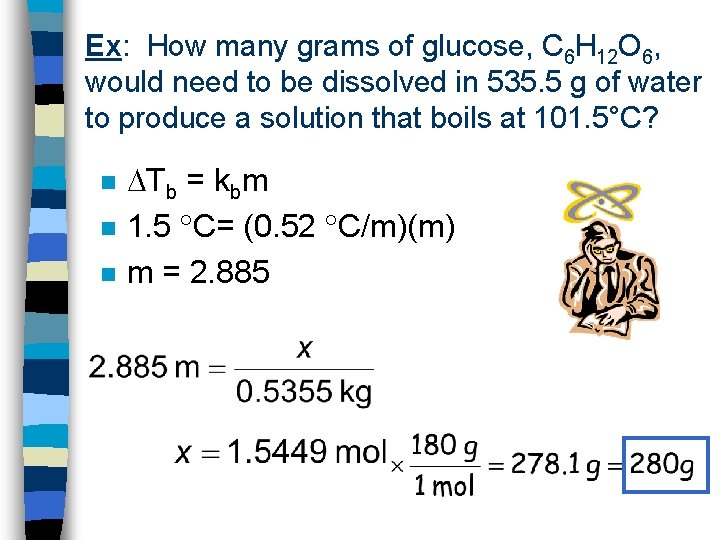
Ex: How many grams of glucose, C 6 H 12 O 6, would need to be dissolved in 535. 5 g of water to produce a solution that boils at 101. 5°C? n n n Tb = kbm 1. 5 C= (0. 52 C/m)(m) m = 2. 885

Freezing/Melting Point Depression n The freezing point of a solution is always lower than that of the pure solvent.

Freezing Point Depression




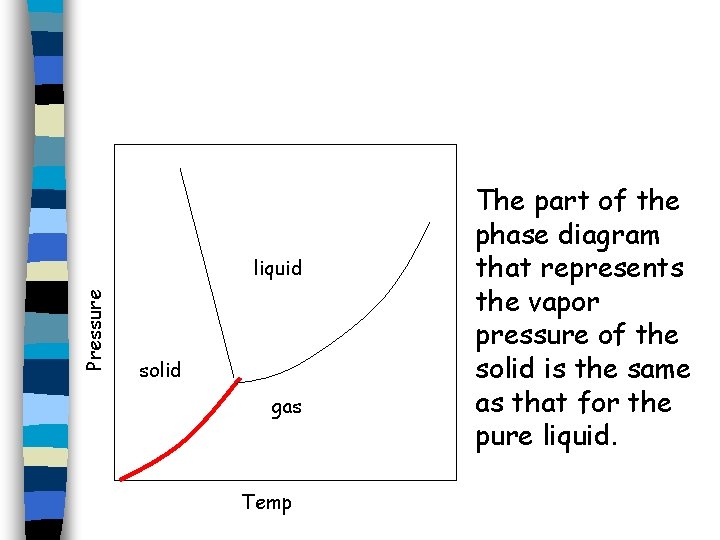
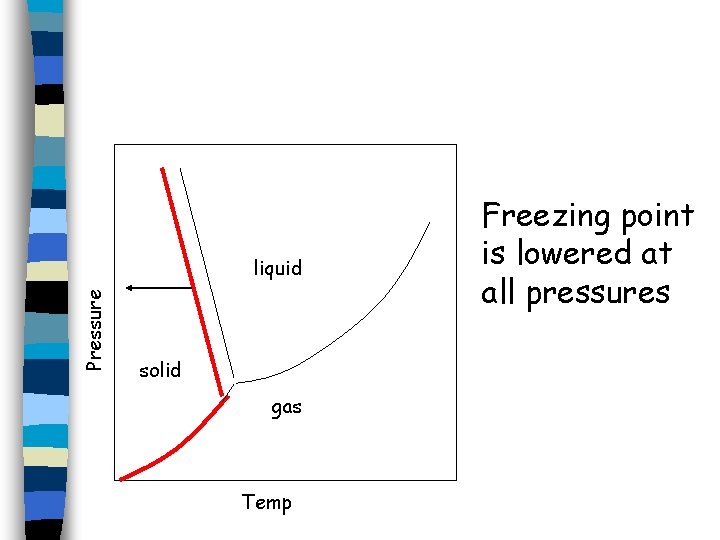
When a solution freezes, crystals of pure solvent usually separate out; the solute molecules are not normally soluble in the solid phase of the solvent. As a result, the part of the phase diagram that represents the vapor pressure of the solid is the same as that for the pure liquid. The vapor pressure curves for the liquid and solid phases meet at the triple point.

Pressure liquid solid gas Temp The part of the phase diagram that represents the vapor pressure of the solid is the same as that for the pure liquid.

Pressure liquid solid gas Temp Freezing point is lowered at all pressures

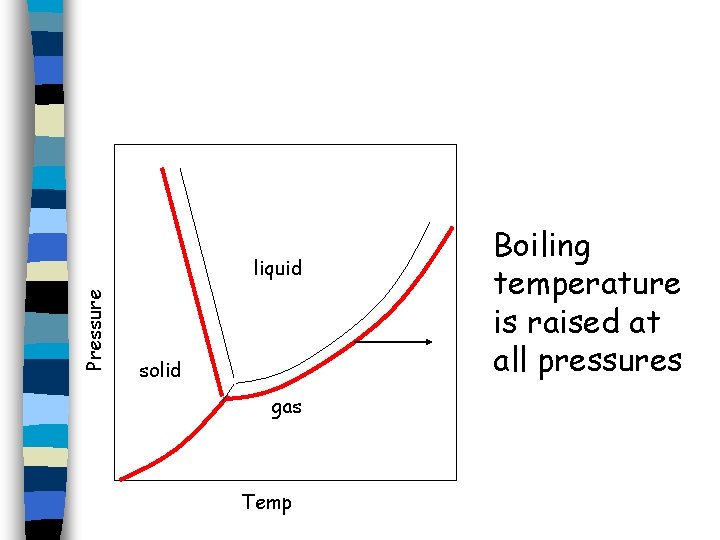
Pressure liquid solid gas Temp Boiling temperature is raised at all pressures

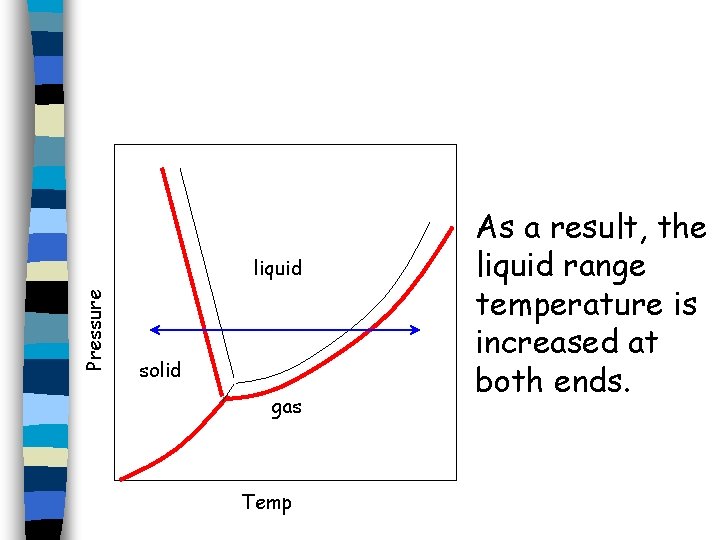
Pressure liquid solid gas Temp As a result, the liquid range temperature is increased at both ends.

Freezing/Melting Point Depression n Tf where: = k fm Tf = lowering of freezing point m = molality of solute kf = the freezing pt depression constant n kf for water = 1. 86 °C/m

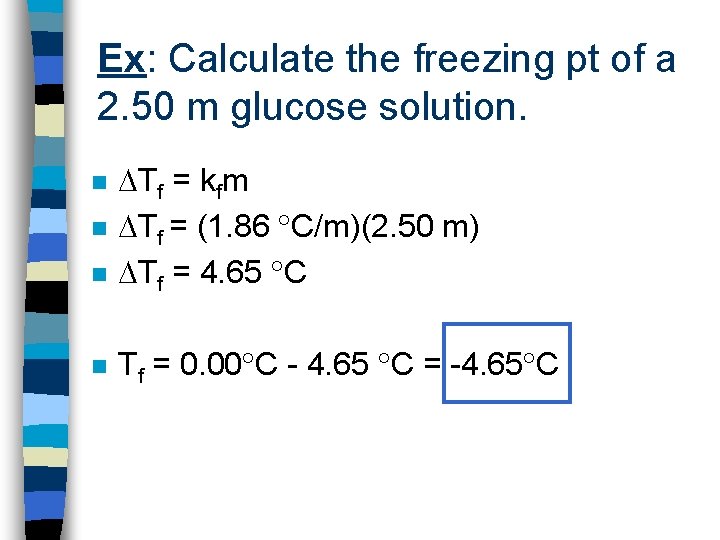
Ex: Calculate the freezing pt of a 2. 50 m glucose solution. n Tf = kfm Tf = (1. 86 C/m)(2. 50 m) Tf = 4. 65 C n Tf = 0. 00 C - 4. 65 C = -4. 65 C n n

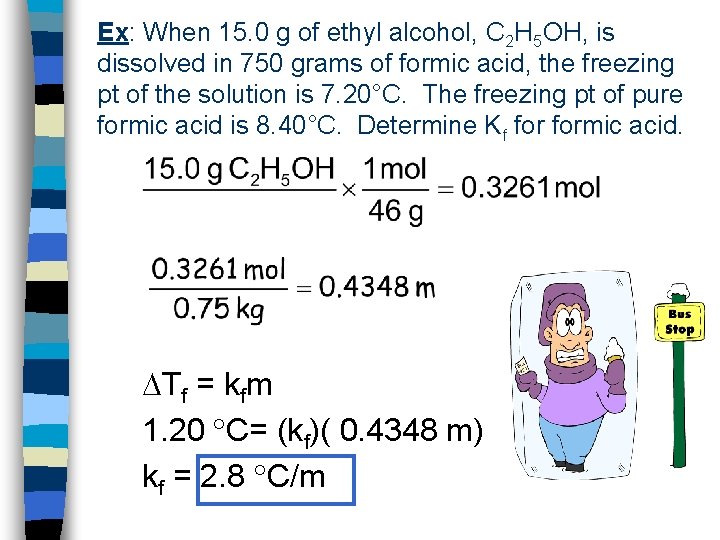
Ex: When 15. 0 g of ethyl alcohol, C 2 H 5 OH, is dissolved in 750 grams of formic acid, the freezing pt of the solution is 7. 20°C. The freezing pt of pure formic acid is 8. 40°C. Determine Kf formic acid. Tf = kfm 1. 20 C= (kf)( 0. 4348 m) kf = 2. 8 C/m

Ex: An antifreeze solution is prepared containing 50. 0 cm 3 of ethylene glycol, C 2 H 6 O 2, (d = 1. 12 g/cm 3), in 50. 0 g water. Calculate the freezing point of this 50 -50 mixture. Would this antifreeze protect a car in Chicago on a day when the temperature gets as low as – 10° F? ( -10 °F = -23. 3° C) Tf = kfm Tf = (1. 86 C/m)(18. 1 m) Tf = 33. 7 C Tf = 0 C – 33. 7 C = -33. 7 C YES!

Electrolytes and Colligative Properties • Colligative properties depend on the # of particles present in solution. • Because ionic solutes dissociate into ions, they have a greater effect on freezing pt and boiling pt than molecular solids of the same molal conc.

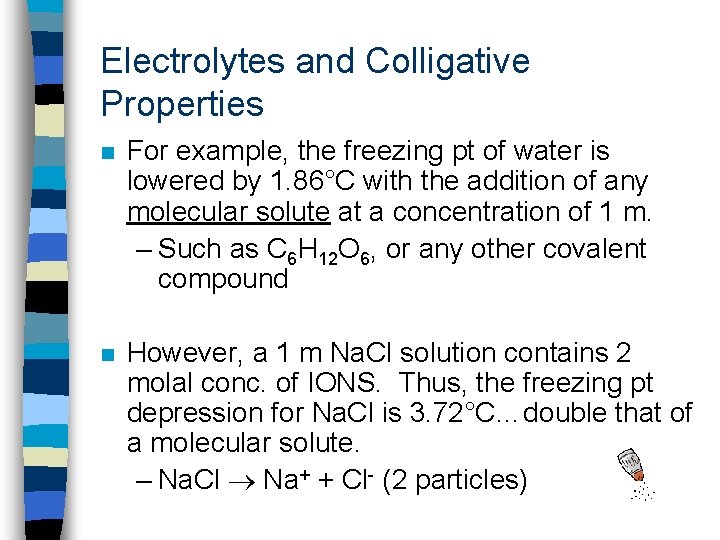
Electrolytes and Colligative Properties n For example, the freezing pt of water is lowered by 1. 86°C with the addition of any molecular solute at a concentration of 1 m. – Such as C 6 H 12 O 6, or any other covalent compound n However, a 1 m Na. Cl solution contains 2 molal conc. of IONS. Thus, the freezing pt depression for Na. Cl is 3. 72°C…double that of a molecular solute. – Na. Cl Na+ + Cl- (2 particles)

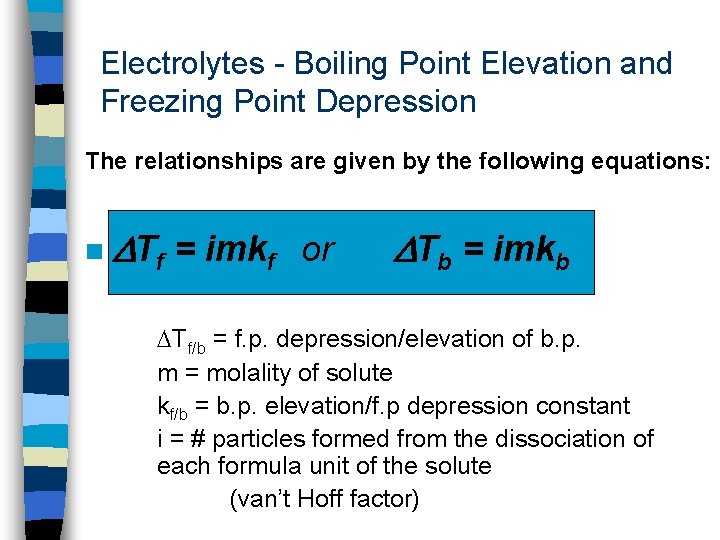
Electrolytes - Boiling Point Elevation and Freezing Point Depression The relationships are given by the following equations: n Tf = imkf or Tb = imkb Tf/b = f. p. depression/elevation of b. p. m = molality of solute kf/b = b. p. elevation/f. p depression constant i = # particles formed from the dissociation of each formula unit of the solute (van’t Hoff factor)

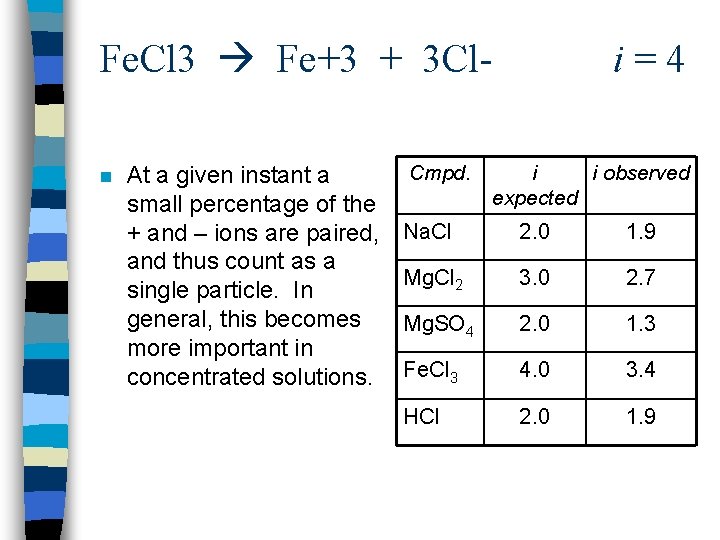
Jacobus. Van’t Hoff (1852 – 1911) n i = The number of particles that the solute molecule breaks into the Van’t Hoff factor n Assumes that when a salt dissolves, it completely dissociates into its component ions, which then move around independently. This assumption is not always true because of ion pairing. n Van’t Hoff a Dutch Chemist received the first Nobel Prize in Chemistry in 1901

Fe. Cl 3 Fe+3 + 3 Cl- i = 4 n At a given instant a small percentage of the + and – ions are paired, and thus count as a single particle. In general, this becomes more important in concentrated solutions. Cmpd. i i observed expected Na. Cl 2. 0 1. 9 Mg. Cl 2 3. 0 2. 7 Mg. SO 4 2. 0 1. 3 Fe. Cl 3 4. 0 3. 4 HCl 2. 0 1. 9

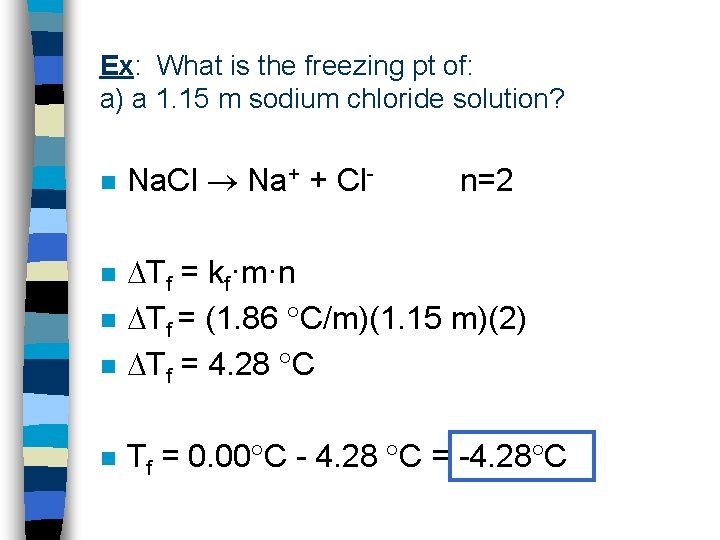
Ex: What is the freezing pt of: a) a 1. 15 m sodium chloride solution? n Na. Cl Na+ + Cl- n=2 n Tf = kf·m·n Tf = (1. 86 C/m)(1. 15 m)(2) Tf = 4. 28 C n Tf = 0. 00 C - 4. 28 C = -4. 28 C n n

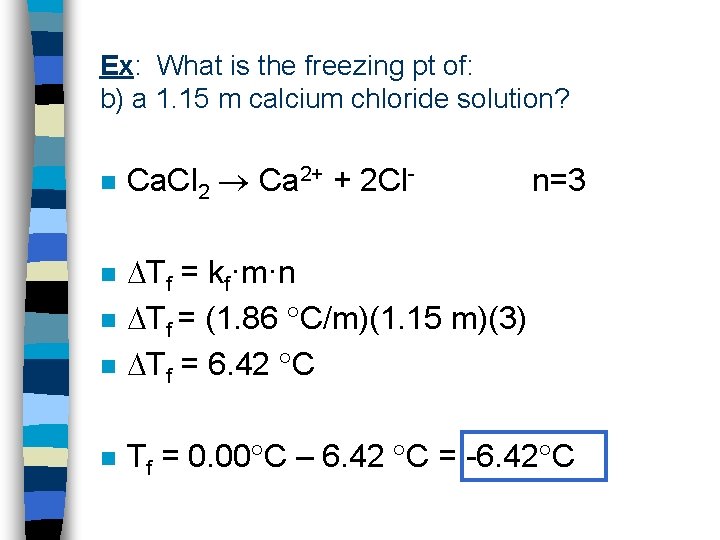
Ex: What is the freezing pt of: b) a 1. 15 m calcium chloride solution? n Ca. Cl 2 Ca 2+ + 2 Cl- n=3 n Tf = kf·m·n Tf = (1. 86 C/m)(1. 15 m)(3) Tf = 6. 42 C n Tf = 0. 00 C – 6. 42 C = -6. 42 C n n

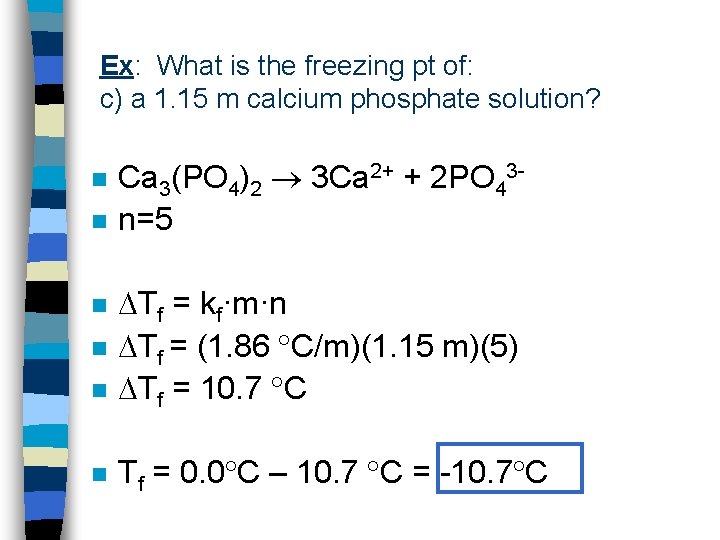
Ex: What is the freezing pt of: c) a 1. 15 m calcium phosphate solution? n n Ca 3(PO 4)2 3 Ca 2+ + 2 PO 43 n=5 n Tf = kf·m·n Tf = (1. 86 C/m)(1. 15 m)(5) Tf = 10. 7 C n Tf = 0. 0 C – 10. 7 C = -10. 7 C n n

Determining Molecular Weights by Freezing Point Depression

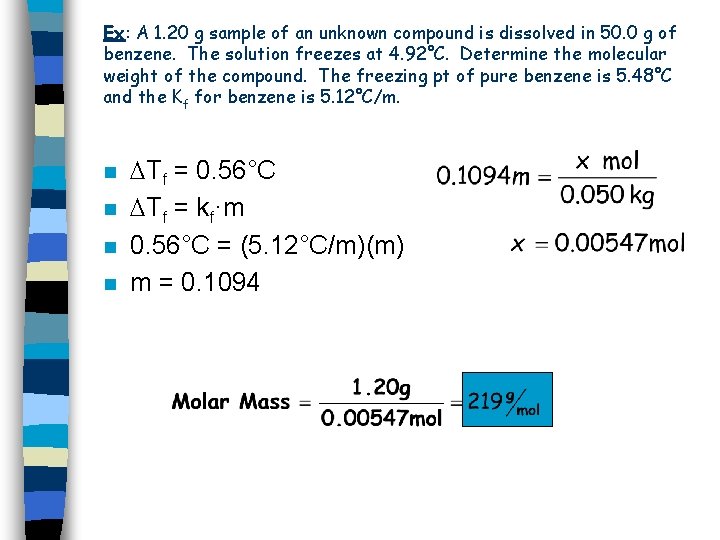
Ex: A 1. 20 g sample of an unknown compound is dissolved in 50. 0 g of benzene. The solution freezes at 4. 92°C. Determine the molecular weight of the compound. The freezing pt of pure benzene is 5. 48°C and the Kf for benzene is 5. 12°C/m. n n Tf = 0. 56°C Tf = kf·m 0. 56°C = (5. 12°C/m)(m) m = 0. 1094

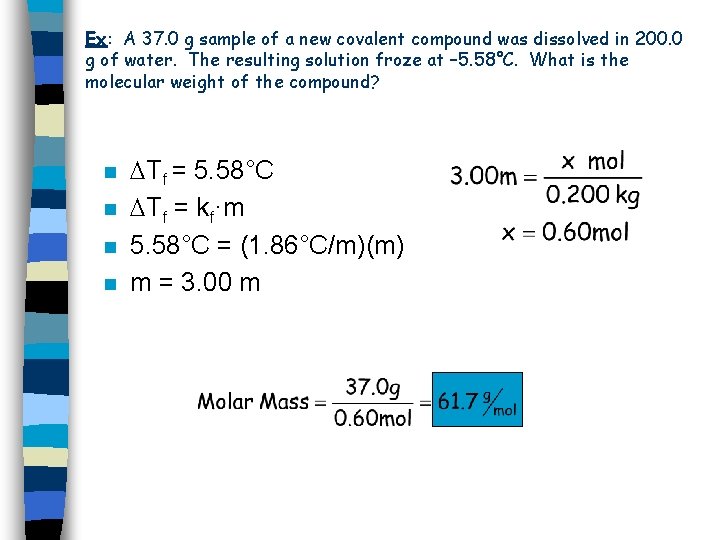
Ex: A 37. 0 g sample of a new covalent compound was dissolved in 200. 0 g of water. The resulting solution froze at – 5. 58°C. What is the molecular weight of the compound? n n Tf = 5. 58°C Tf = kf·m 5. 58°C = (1. 86°C/m)(m) m = 3. 00 m

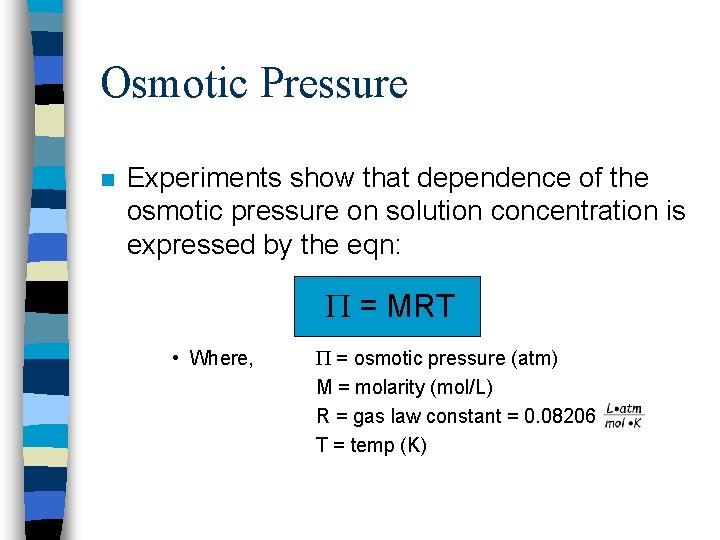
Osmotic Pressure n Experiments show that dependence of the osmotic pressure on solution concentration is expressed by the eqn: = MRT • Where, = osmotic pressure (atm) M = molarity (mol/L) R = gas law constant = 0. 08206 T = temp (K)

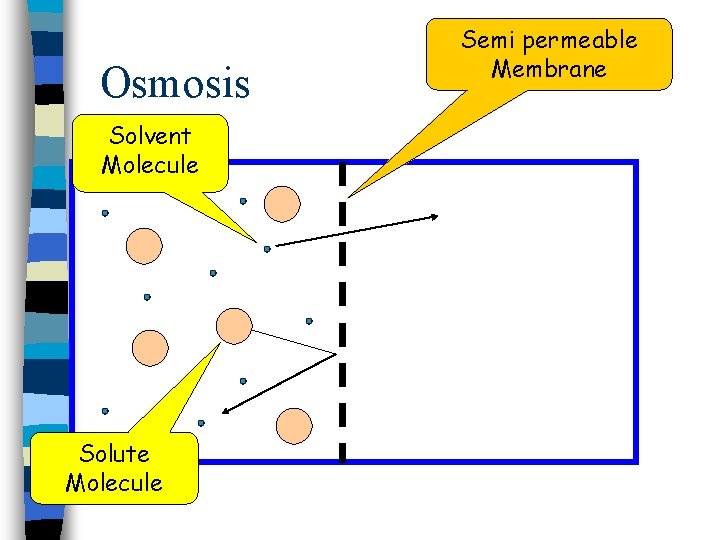
Osmosis Solvent Molecule Solute Molecule Semi permeable Membrane

Water Flow

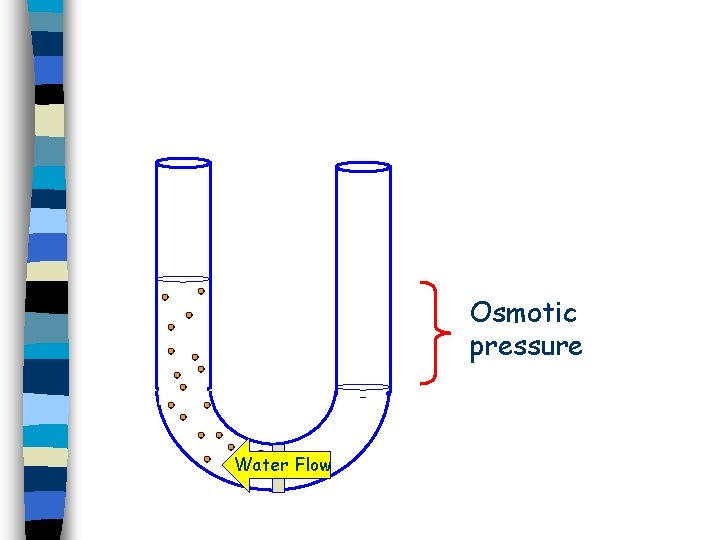
Osmotic pressure Water Flow

A simple rule to remember is: Salt is a solute, when it is concentrated inside or outside the cell, it will draw the water in its direction. This is also why you get thirsty after eating something salty.

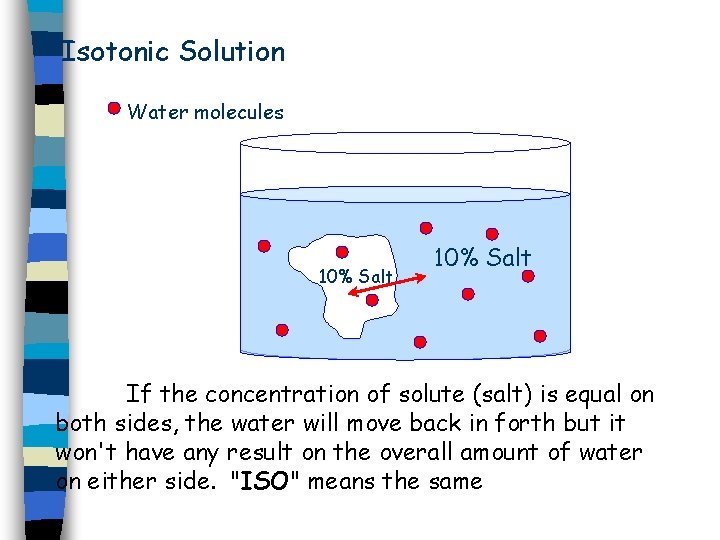
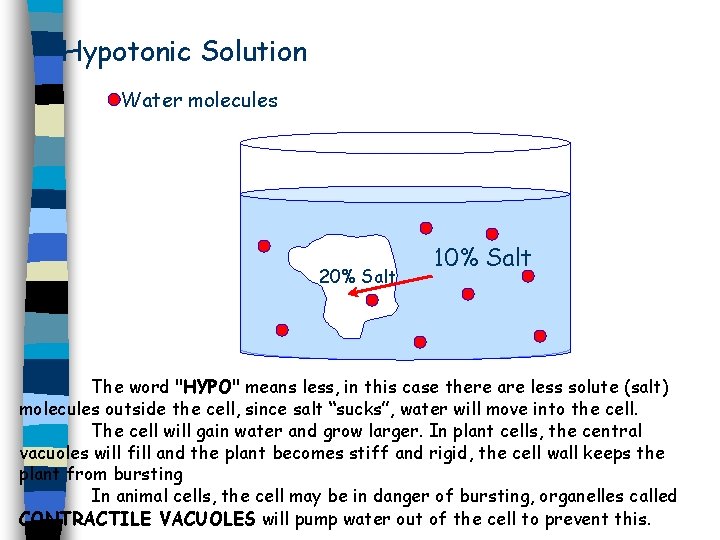
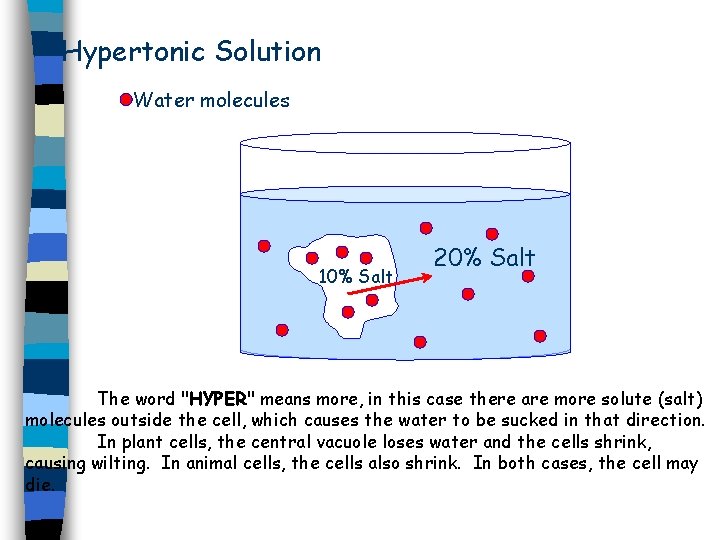
Type of Solutions 1. Isotonic: Solution has the same solute concentration as the cell. 2. Hypotonic: Solution has less solute concentration than the cell. 3. Hypertonic: Solution has more solute concentration the cell.

Isotonic Solution Water molecules 10% Salt If the concentration of solute (salt) is equal on both sides, the water will move back in forth but it won't have any result on the overall amount of water on either side. "ISO" means the same

Hypotonic Solution Water molecules 20% Salt 10% Salt The word "HYPO" means less, in this case there are less solute (salt) molecules outside the cell, since salt “sucks”, water will move into the cell. The cell will gain water and grow larger. In plant cells, the central vacuoles will fill and the plant becomes stiff and rigid, the cell wall keeps the plant from bursting In animal cells, the cell may be in danger of bursting, organelles called CONTRACTILE VACUOLES will pump water out of the cell to prevent this.

Hypertonic Solution Water molecules 10% Salt 20% Salt The word "HYPER" means more, in this case there are more solute (salt) molecules outside the cell, which causes the water to be sucked in that direction. In plant cells, the central vacuole loses water and the cells shrink, causing wilting. In animal cells, the cells also shrink. In both cases, the cell may die.

This is why it is dangerous to drink sea water - its a myth that drinking sea water will cause you to go insane, but people marooned at sea will speed up dehydration (and death) by drinking sea water. This is also why "salting fields" was a common tactic during war, it would kill the crops in the field, thus causing food shortages.

Animation of cells placed in various solutions (wait for it to play…)