Elements Compounds Mixtures GOAL To tell the difference





















- Slides: 26

Elements, Compounds & Mixtures

GOAL • To tell the difference between elements, compounds and mixtures and to give examples of each

Matter • Matter is anything that has mass and takes up space. • Matter is made up of many different things:

Substance A substance is a single kind of matter that has a specific composition and a specific set of properties - for example, color, melting point, texture. . .

Physical Properties are characteristics of substances that can be observed without changing it into another substance. Examples: freezing or melting density hardness color

Chemical Properties are characteristics of pure substances that describe their ability to change in to different substances.

Chemical Properties Examples: Does it burn? (Flammability) Does it combine with oxygen? (Iron does and forms rust. ) What will the substance react with?

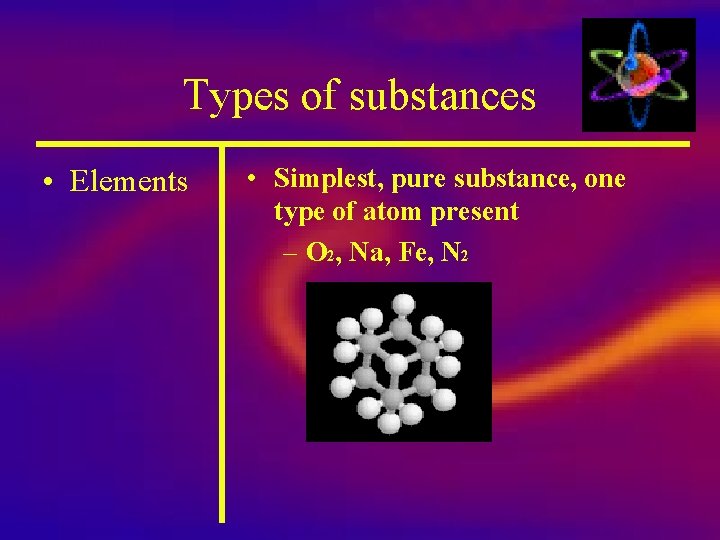
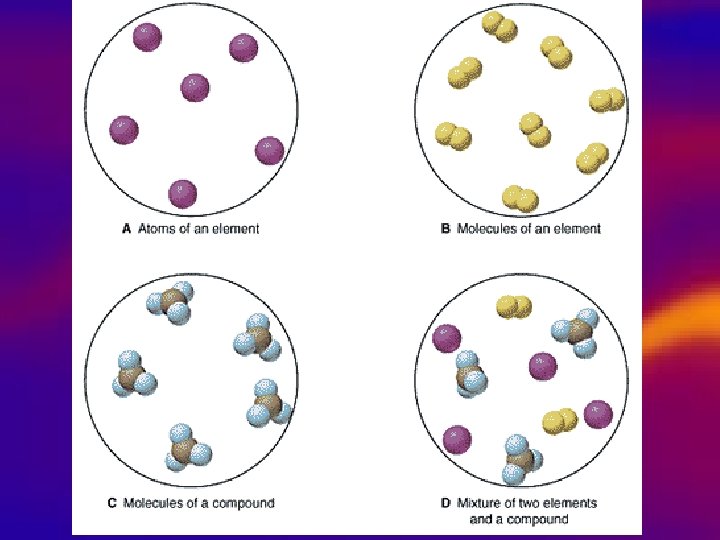
Types of substances • Elements • Simplest, pure substance, one type of atom present – O 2, Na, Fe, N 2


Elements There are over 100 elements in the periodic table. Elements all have unique symbols: Hydrogen: Lithium : Oxygen : Neon : H Li O 2 Ne

Types of substances • Compound • 1. Made of several different atoms (combined by chemical reaction) – Salt- Na. Cl – Sugar- C 6 H 12 O 6 • 2. Different properties from the elements that make them up

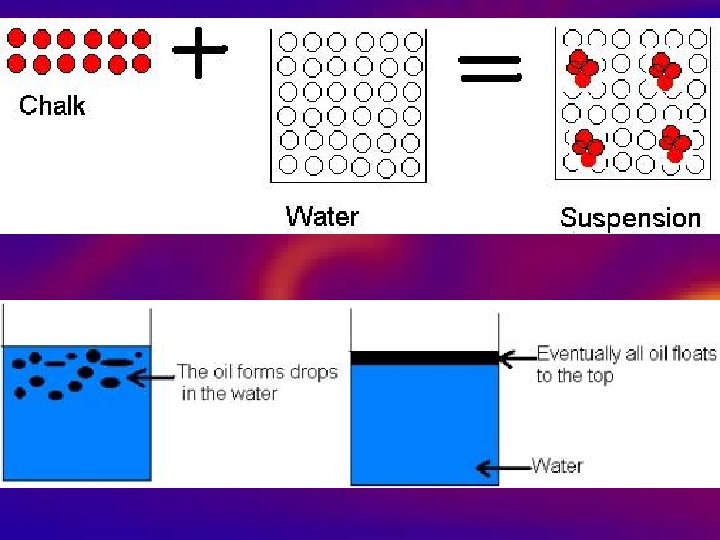
Types of substances • Mixtures • 1. Made of more than one kind of molecule (many compounds) • 2. Can be separated or pulled apart by physically (they are NOT chemically combined) • 3. 2 types: – Heterogeneous – Homogeneous

Heterogeneous Mixtures • You can see the different parts. • • Examples: Salad Soil Bowl of Mixed Nuts

Types of substances • Heterogeneous • 1. Not the same throughout Mixtures • 2. Large easily seen particles that can be separated out • 3. Heterogeneous liquid mixtures known as suspensions – Settles after standing • 4. Tacos, salad, italian dressing, pebble/sand mixture



Homogeneous Mixture • They are so evenly mixed, you can not see the individual parts. • Examples: • Salt dissolved in water • Brass is a mixture of copper and zinc

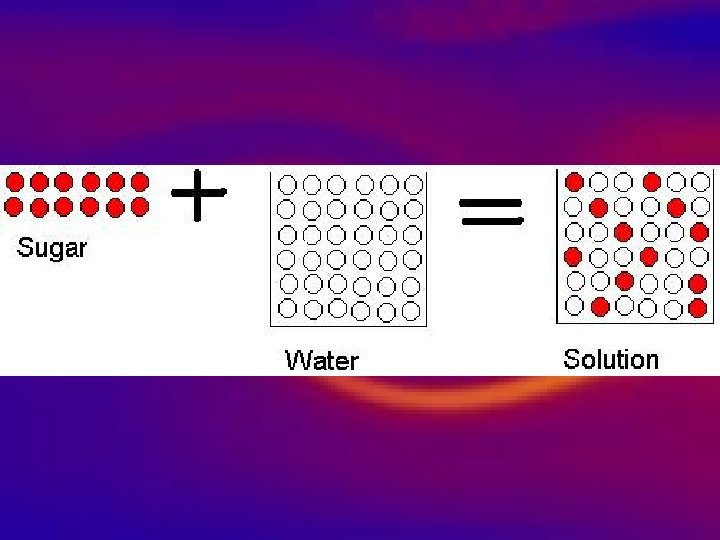
Types of substances • Homogeneous • 1. Appears same throughout • 2. Well mixed Mixtures • 3. Won’t settle upon standing, • 4. Tiny unrecognizable particles – Solutions are homogeneous mixtures

Types of substances • Homogeneous Mixtures – Won’t settle out – Cloudy-Scatters light – Particles mix, don’t dissolve • Milk, lotion, toothpaste


Types of substances • Homogeneous • Mixtures Metal Solutions a) Alloys- solid metal solutions b) Gold= copper and gold c) Brass= copper and zinc d) Sterling silver= silver and copper

Types of substances • Homogeneous • Mixtures Solutions a) One substance dissolved in another b) Tiny particles can’t be seen c) Best mixed d) Clear or transparent- light passes right through e) Ocean water, lemonade, air



