Separating Mixtures Two types of mixtures Homogeneous mixtures














- Slides: 17

Separating Mixtures

Two types of mixtures • Homogeneous mixtures – where everything looks the same but is made up of two or more types of pure substances – SOLUTIONS! – Milk, juice, salt and water • Heterogeneous mixtures – where we can spot the difference with our eyes between particles – Gravel, lego – We usually refer to them as mechanical mixtures

Hand Separation • Using our hands or tools that we use with hands • Sieve, magnets, or our hands • Separating by particle size

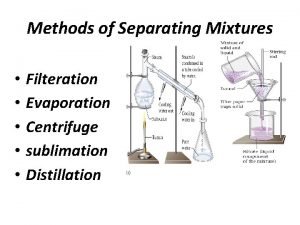
Filtration • Similar to sieve but for finer particles. • Usually for small particles formed in a solution – Precipitate

Evaporation • Between a solid and liquid • We evaporate the liquid and have the solid left behind • Think of sand water • Evaporate the water, get left with the sand • We use the boiling point of the liquid to evaporate it.

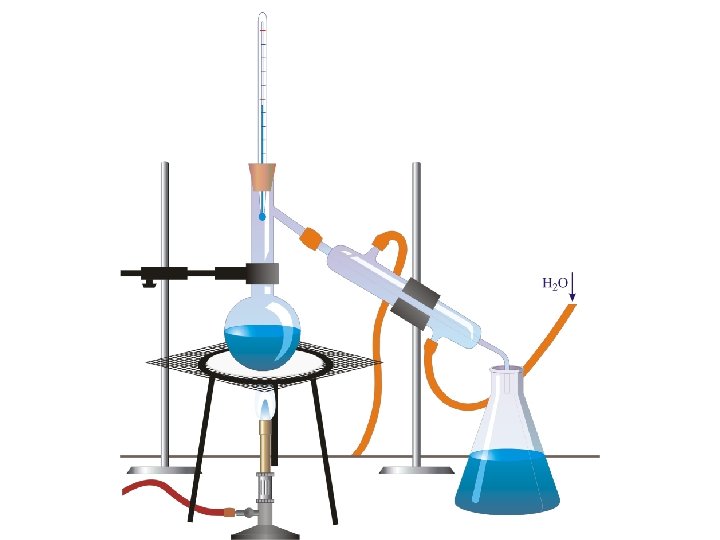
Distillation • Between a liquid and a liquid. • Similar to evaporation but between the boiling points of two liquids • Example – Water has a boiling point of 100°C – Methanol has a boiling point of 64°C – We can heat up the mixture to 70°C and let the methanol boil and leave the water behind.


Solvent Extraction • Using the information about solvents dissolving certain solids • Sugar and sand – Sugar dissolves in water – Sand does not dissolve in water – Pour water in, dissolve the sugar and collect the sand.

Solvent Extraction • • Same can be done to liquids and liquids Some liquids mix Some liquids do not mix “Like dissolves like” Polar dissolves polar Non-polar dissolves non-polar Do not worry about the last 2 points as much.

• Oil (non-polar) and water (polar) do not mix • We call them immiscible. When liquids do not mix • Two liquids that mix (methanol and ethanol) we say they are miscible • In order to separate, we let the liquids sit and the denser one will usually sink. We can use a funnel to remove.

Recrystallization • Similar to evaporation, but inside a solution. • Remember a solution is a HOMOGENEOUS MIXTURE! So we might not know there are solids inside. • We heat up the solution and allow the liquid to evaporate. The solid will recrystallize again • Water and salt

Gravity Separation • • • We let gravity separate it Density is involved Denser substances sink Less dense substances float We can speed this up using a centrifuge

Video

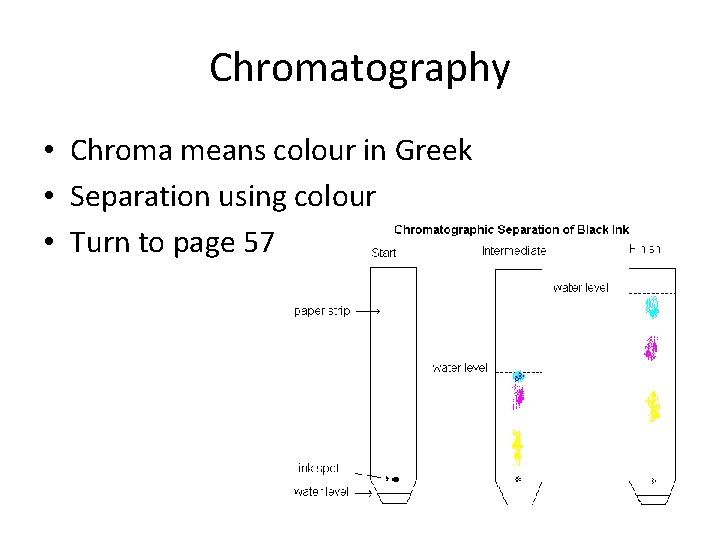
Chromatography • Chroma means colour in Greek • Separation using colour • Turn to page 57

Video!

Pg 58 Separation Table

Homework • Pg 58 • #45 -47 • #54 and 58
 Explain separating funnel
Explain separating funnel Application of homogeneous differential equation
Application of homogeneous differential equation Separating mixtures grade 7
Separating mixtures grade 7 Filtration is used to separate
Filtration is used to separate Evaporation mixtures
Evaporation mixtures Example of magnetism mixture
Example of magnetism mixture Natural science grade 7 term 2 practical tasks
Natural science grade 7 term 2 practical tasks Pure substance
Pure substance Centrifugation separating mixtures
Centrifugation separating mixtures How would you separate salt and sand
How would you separate salt and sand Separation techniques gcse
Separation techniques gcse Separating mixtures simulation
Separating mixtures simulation Separating rock salt bbc bitesize
Separating rock salt bbc bitesize Separating mechanical mixtures
Separating mechanical mixtures 10 methods of separating mixtures
10 methods of separating mixtures Different methods of separating mixtures
Different methods of separating mixtures Objectives in separating mixtures
Objectives in separating mixtures Example of magnetism in separating mixtures
Example of magnetism in separating mixtures