Separation of Mixtures Mixtures Types of mixtures Homogeneous







- Slides: 15

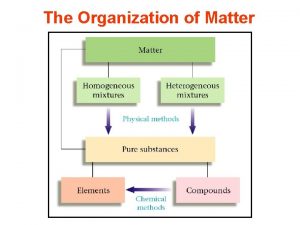
Separation of Mixtures

Mixtures Types of mixtures: Homogeneous- The composition of the mixture is the same throughout and has uniform properties. (air, steel, sugar water) Heterogeneous- Components of the mixture are not uniform and have different properties within the phases. (oil and water, milk and cereal, sand water)

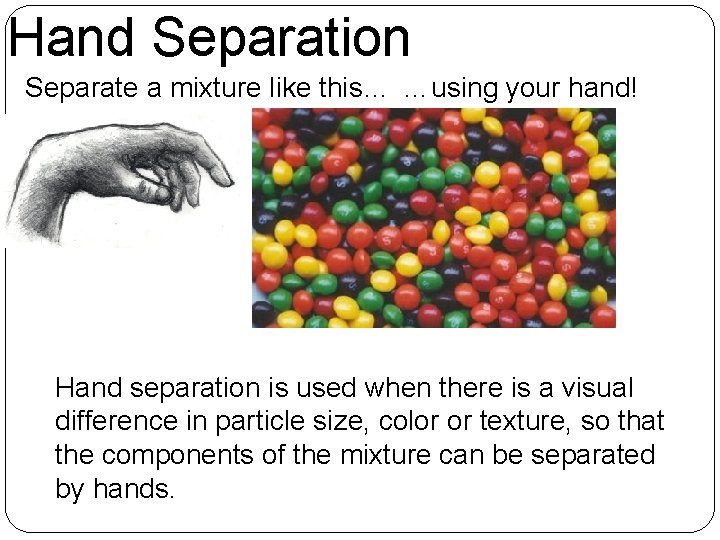
Hand Separation Separate a mixture like this… …using your hand! Hand separation is used when there is a visual difference in particle size, color or texture, so that the components of the mixture can be separated by hands.

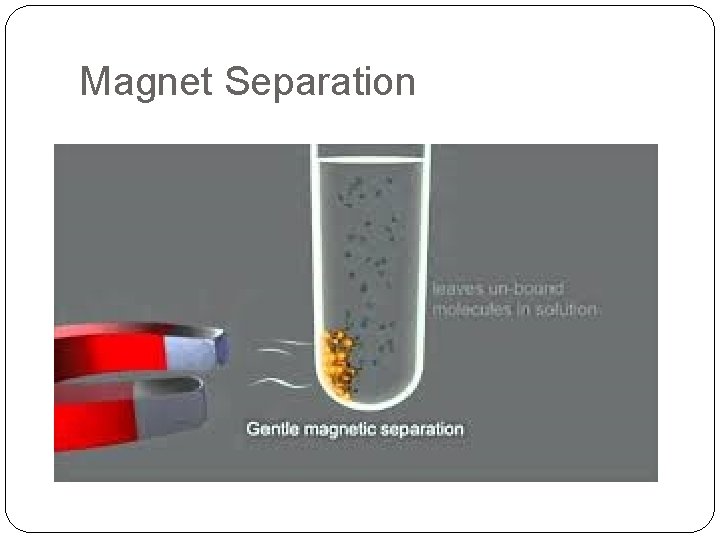
Magnet Separation

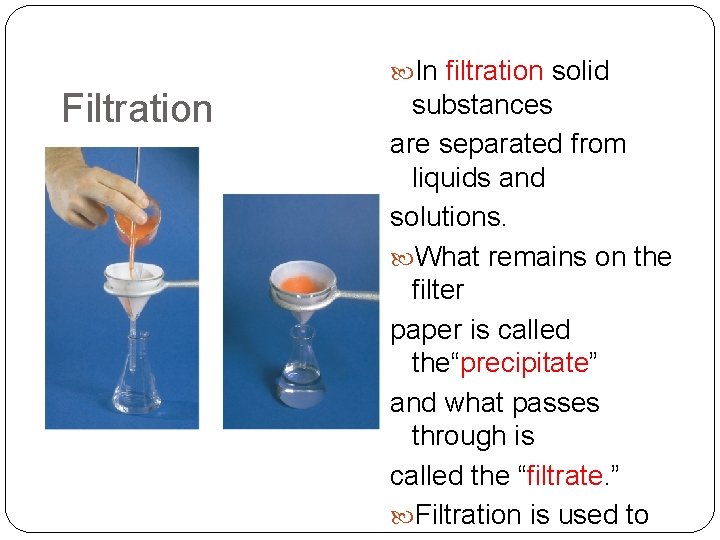
Filtration In filtration solid substances are separated from liquids and solutions. What remains on the filter paper is called the“precipitate” and what passes through is called the “filtrate. ” Filtration is used to

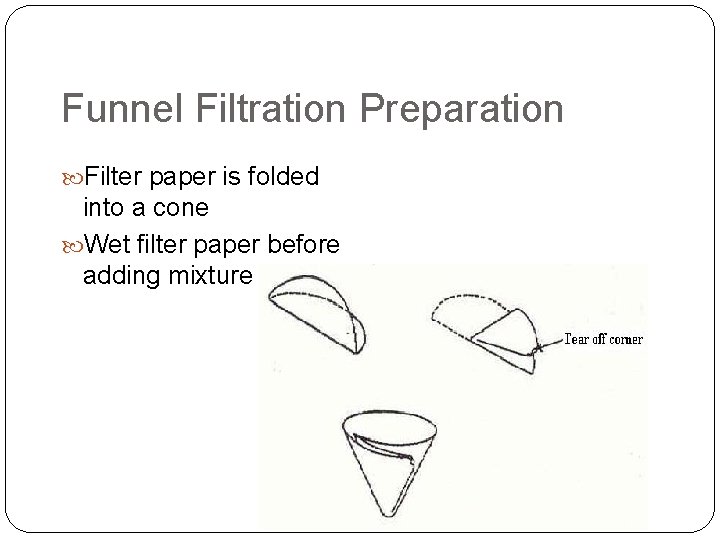
Funnel Filtration Preparation Filter paper is folded into a cone Wet filter paper before adding mixture

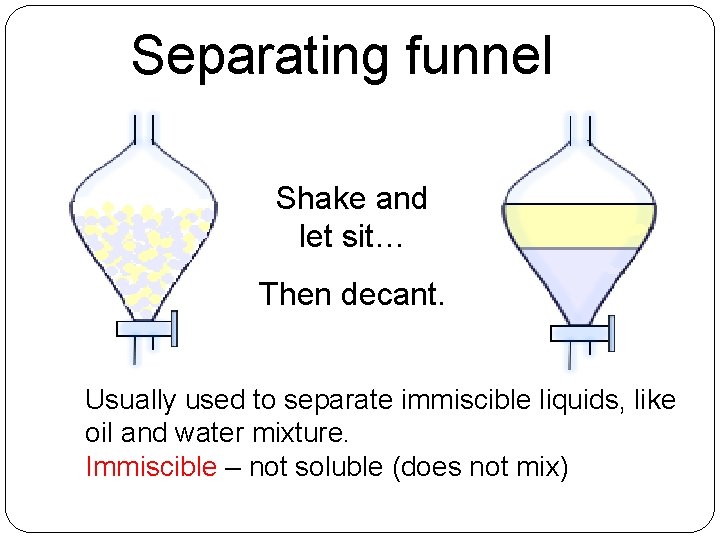
Separating funnel Shake and let sit… Then decant. Usually used to separate immiscible liquids, like oil and water mixture. Immiscible – not soluble (does not mix)

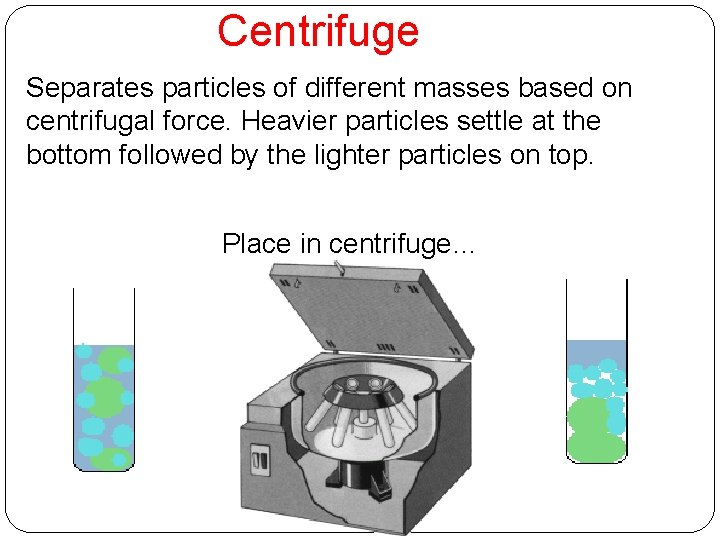
Centrifuge Separates particles of different masses based on centrifugal force. Heavier particles settle at the bottom followed by the lighter particles on top. Place in centrifuge…

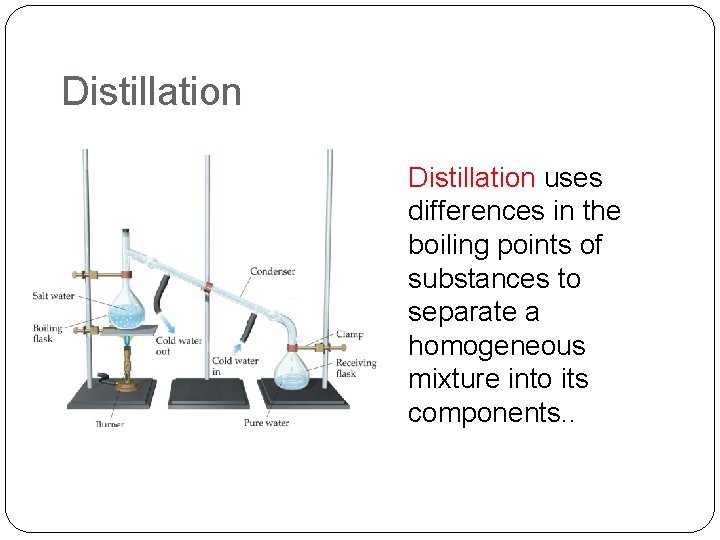
Distillation uses differences in the boiling points of substances to separate a homogeneous mixture into its components. .

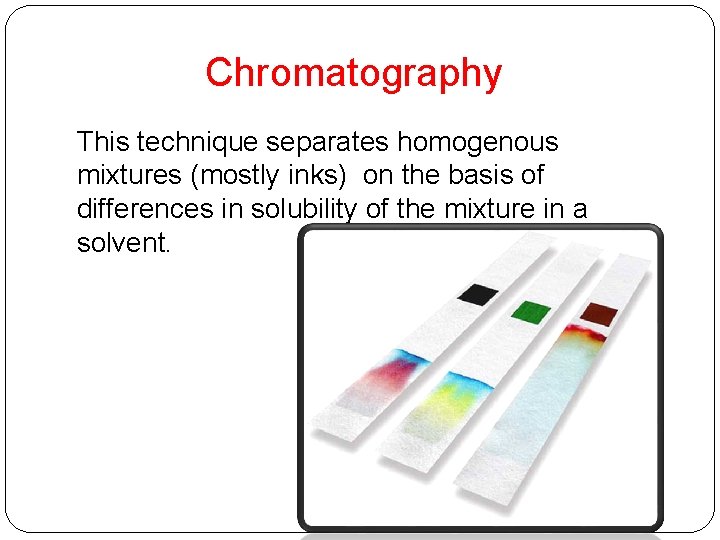
Chromatography This technique separates homogenous mixtures (mostly inks) on the basis of differences in solubility of the mixture in a solvent.

Filtration and Evaporation The liquid (solvent) separates from the solution will evaporate leaving behind any dissolved particles (solute).

Today- In Lab Composition Book Purpose: State what you are trying to accomplish Materials: List all materials needed Safety: List all precautions that must be taken Procedure: Step by step procedure None of these should be word for word the same (even within groups) Must be approved by Miss Sparks via signature to continue.

Properties and Changes of Matter

Types of Properties Physical Properties… Can be observed without changing a substance into another substance. Boiling point, density, mass, volume, etc. Chemical Properties… Can only be observed when a substance is changed into another substance. Flammability, corrosiveness, reactivity with acid, etc.

Types of Properties Intensive Properties… Are independent of the amount of the substance that is present. Density, boiling point, color, etc. Extensive Properties… Depend upon the amount of the substance present. Mass, volume, energy, etc.
 Non homogeneous differential equation
Non homogeneous differential equation Are all solutions homogeneous mixtures
Are all solutions homogeneous mixtures Different types of mixtures
Different types of mixtures Examples of nonmatter
Examples of nonmatter Common homogeneous mixtures
Common homogeneous mixtures Facts about mixtures
Facts about mixtures Are all aqueous solutions homogeneous
Are all aqueous solutions homogeneous Are aqueous solutions homogeneous mixtures
Are aqueous solutions homogeneous mixtures Are solutions homogeneous
Are solutions homogeneous Separation methods
Separation methods Tyndall effect
Tyndall effect Manual separation of mixtures
Manual separation of mixtures What is evaporation in mixtures
What is evaporation in mixtures Elements compounds and mixtures graphic organizer
Elements compounds and mixtures graphic organizer 6 types of mixtures
6 types of mixtures Different types of mixtures
Different types of mixtures