Honors Chemistry Unit 2 Matter and Energy Guiding























- Slides: 67

Honors Chemistry Unit 2: Matter and Energy

Guiding Questions Why do substances boil or freeze at different temperatures? Why do we put salt on the roads in the winter? Why does sweating cool us? What is energy? How do we measure energy?

Matter Introductory Definitions (…pull out your vocab!) matter: anything having mass and volume mass: the amount of matter in an object weight: the pull of gravity on an object volume: the space an object occupies units: L, dm 3, m. L, cm 3 state of matter: solid, liquid, or gas L 3

Solid, Liquid, Gas (a) Particles in solid (b) Particles in liquid (c) Particles in gas

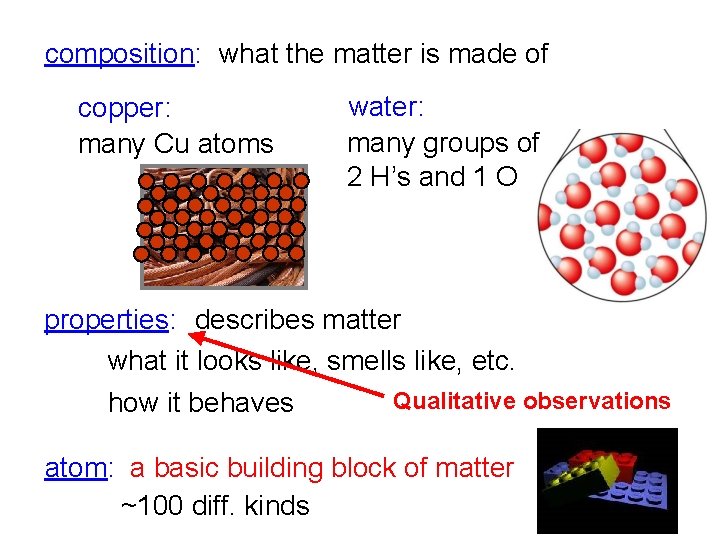
composition: what the matter is made of copper: many Cu atoms water: many groups of 2 H’s and 1 O properties: describes matter what it looks like, smells like, etc. how it behaves Qualitative observations atom: a basic building block of matter ~100 diff. kinds

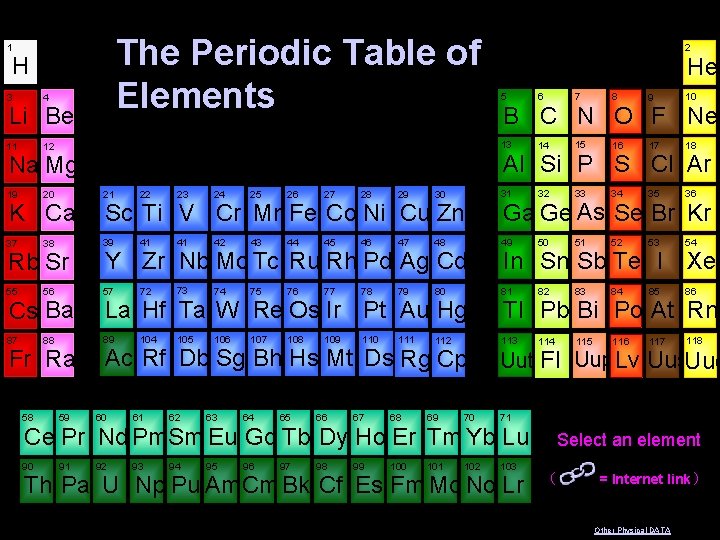
1 The Periodic Table of Elements H 6 7 8 9 10 13 14 15 16 17 18 30 31 32 33 34 35 36 47 48 49 50 51 52 53 54 78 79 80 81 82 83 84 85 86 110 111 112 113 114 115 116 117 118 4 11 12 19 20 21 22 23 24 25 26 27 28 29 37 38 39 41 41 42 43 44 45 46 55 56 57 72 73 74 75 76 77 87 88 89 104 105 106 107 108 109 Rb Sr Cs Ba Fr Ra 58 59 B C N O F Ne Al Si P S Cl Ar Na Mg K Ca He 5 3 Li Be 2 Sc Ti V Cr Mn Fe Co Ni Cu Zn Y Zr Nb Mo Tc Ru Rh Pd Ag Cd La Hf Ta W Re Os Ir Pt Au Hg Ac Rf Db Sg Bh Hs Mt Ds Rg Cp 60 61 62 63 64 65 66 67 68 69 70 Ga Ge As Se Br Kr In Sn Sb Te I Xe Tl Pb Bi Po At Rn Uut Fl Uup. Lv Uus. Uuo 71 Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu 90 91 92 93 94 95 96 97 98 99 100 101 102 103 Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr Select an element ( = Internet link ) Other Physical DATA

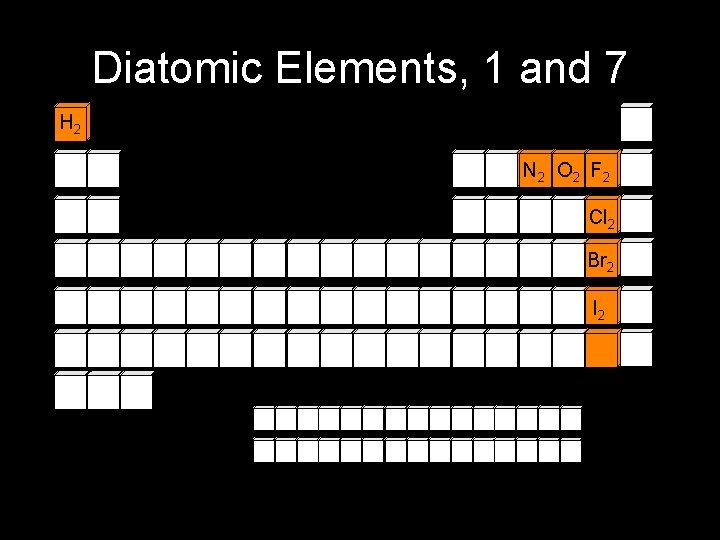
Elements contain only one type of atom 1. monatomic elements consist of unbonded, “like” atoms e. g. , Fe, Al, Cu, He 2. polyatomic elements consist of several “like” atoms bonded together diatomic elements: H 2 O 2 Br 2 F 2 I 2 N 2 Cl 2 “HOBr. FINCl = Hoberfinckle” others: P 4 or S 8 “Br. INCl. HOF = Brinklehoff”

Diatomic Elements, 1 and 7 H 2 N 2 O 2 F 2 Cl 2 Br 2 I 2

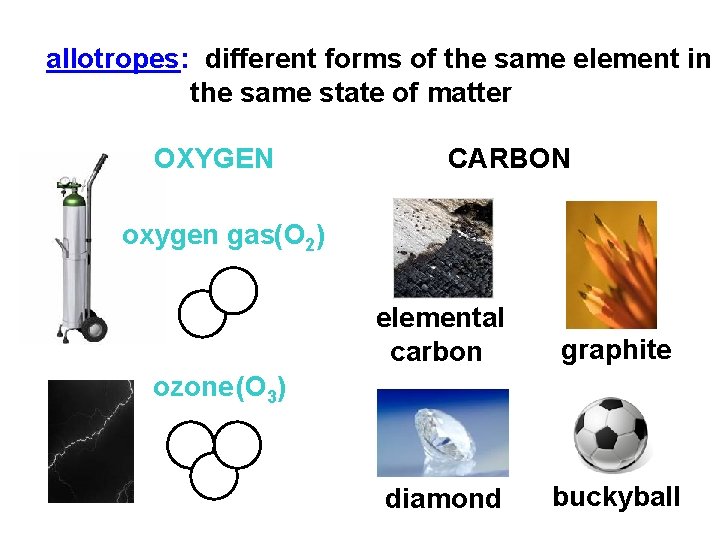
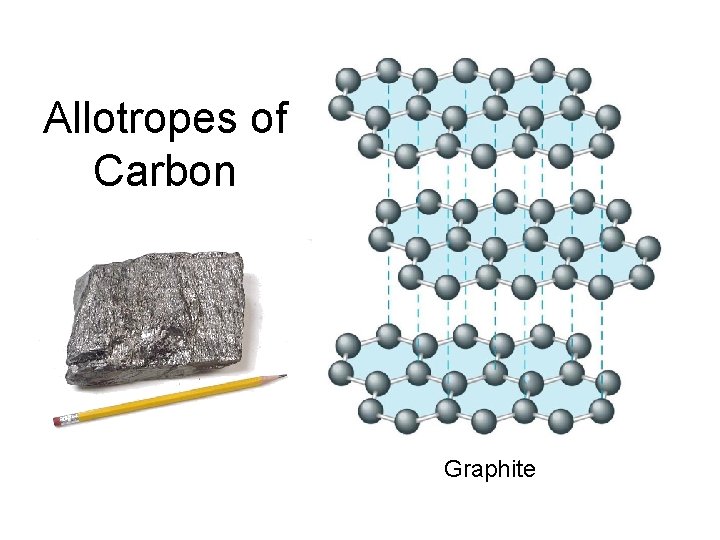
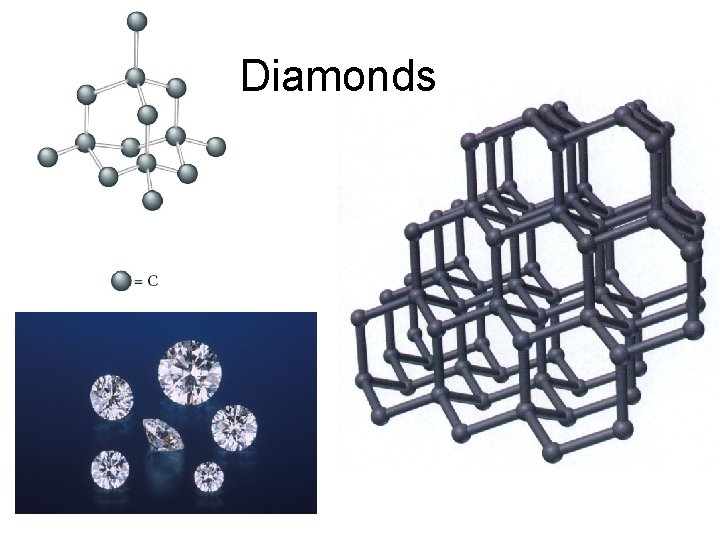
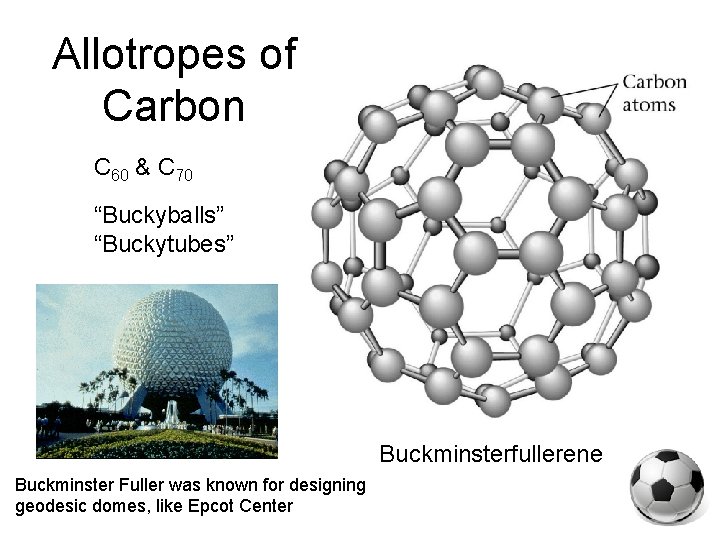
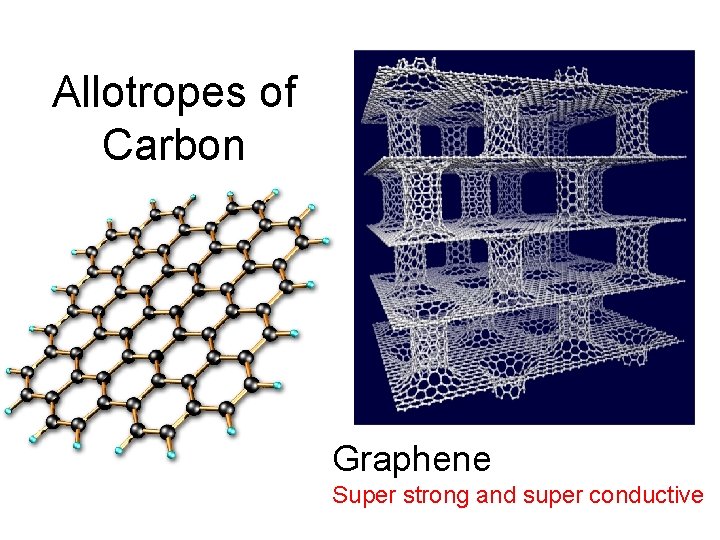
allotropes: different forms of the same element in the same state of matter OXYGEN CARBON oxygen gas(O 2) elemental carbon graphite diamond buckyball ozone (O 3)

Allotropes of Carbon Graphite

Diamonds

Allotropes of Carbon C 60 & C 70 “Buckyballs” “Buckytubes” Buckminsterfullerene Buckminster Fuller was known for designing geodesic domes, like Epcot Center

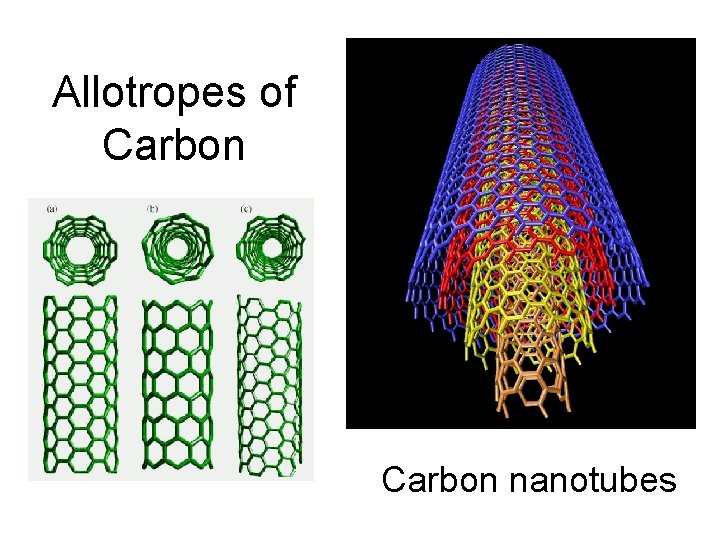
Allotropes of Carbon nanotubes

Allotropes of Carbon Graphene Super strong and super conductive

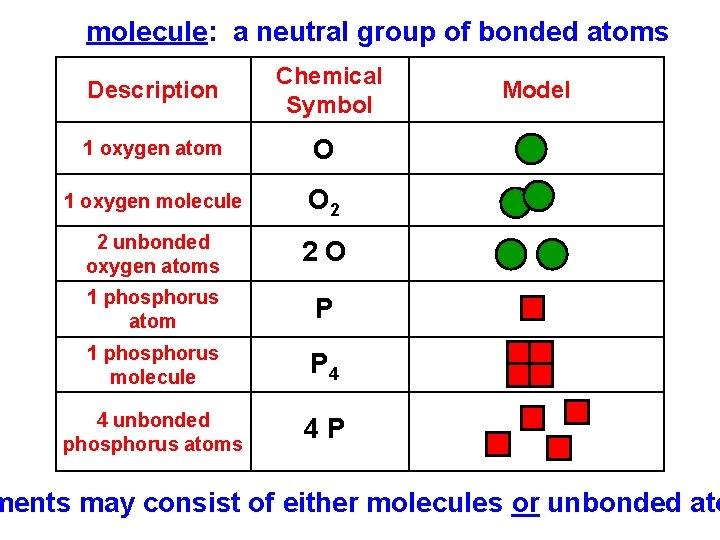
molecule: a neutral group of bonded atoms Description Chemical Symbol 1 oxygen atom O 1 oxygen molecule O 2 2 unbonded oxygen atoms 2 O 1 phosphorus atom P 1 phosphorus molecule P 4 4 unbonded phosphorus atoms 4 P Model ments may consist of either molecules or unbonded ato

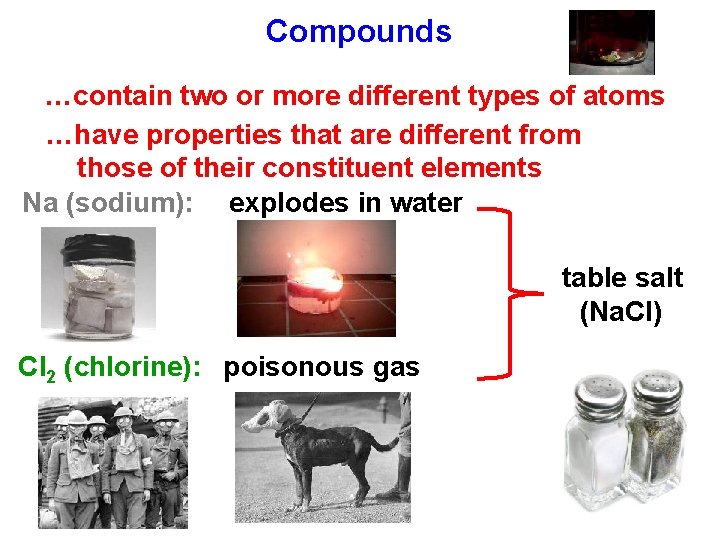
Compounds …contain two or more different types of atoms …have properties that are different from those of their constituent elements Na (sodium): explodes in water table salt (Na. Cl) Cl 2 (chlorine): poisonous gas

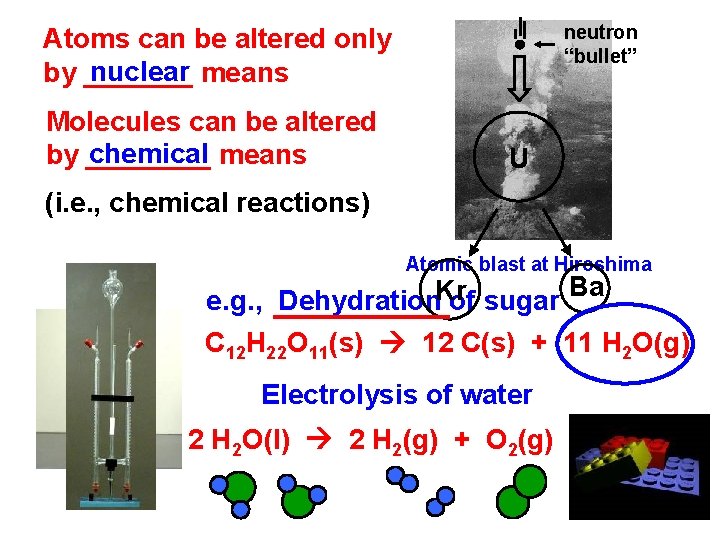
neutron “bullet” Atoms can be altered only nuclear means by _______ Molecules can be altered chemical means by ____ U (i. e. , chemical reactions) Atomic blast at Hiroshima e. g. , Dehydration. Kr of sugar Ba C 12 H 22 O 11(s) 12 C(s) + 11 H 2 O(g) Electrolysis of water 2 H 2 O(l) 2 H 2(g) + O 2(g)

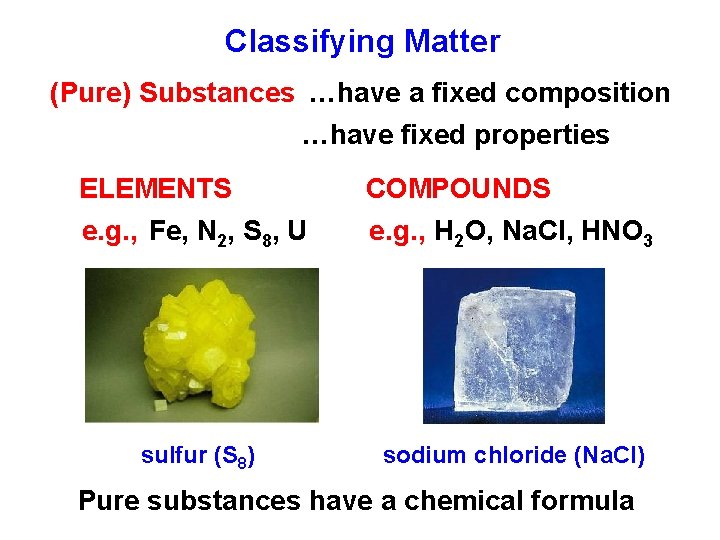
Classifying Matter (Pure) Substances …have a fixed composition …have fixed properties ELEMENTS COMPOUNDS e. g. , Fe, N 2, S 8, U e. g. , H 2 O, Na. Cl, HNO 3 sulfur (S 8) sodium chloride (Na. Cl) Pure substances have a chemical formula

Mixtures two or more substances mixed together …have varying composition …have varying properties The substances are NOT chemically bonded, and they… retain their individual properties Tea, orange juice, oceans, and air are all mixtures

Two Types of Mixtures 1. homogeneous: (or solution) particles are microscopic; sample has the same composition and properties throughout; evenly mixed Oh Yeah! e. g. , salt water Kool Aid alloy: a homogeneous mixture of metals e. g. , bronze (Cu + Sn) pewter (Pb + Sn) brass (Cu + Zn)

Two Types of Mixtures (cont. ) 2. heterogeneous: different composition and properties in the sample; unevenly mixed tossed salad e. g. , raisin bran suspension: settles over time e. g. , paint snowy-bulb gifts

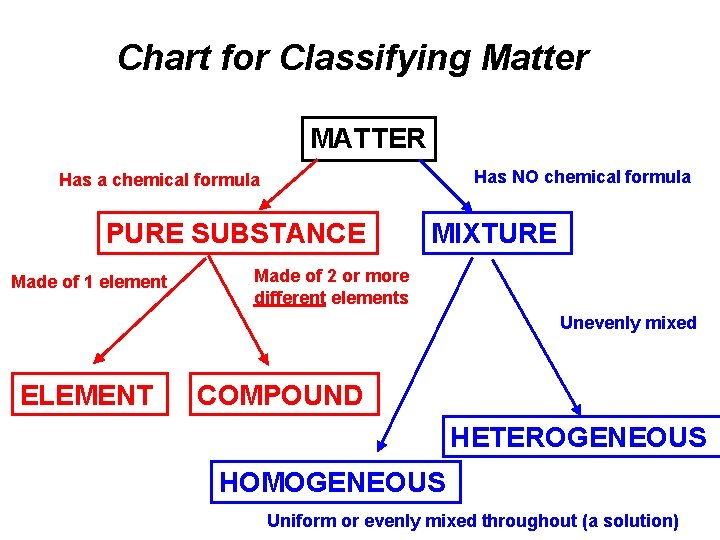
Chart for Classifying Matter MATTER Has NO chemical formula Has a chemical formula PURE SUBSTANCE Made of 1 element MIXTURE Made of 2 or more different elements Unevenly mixed ELEMENT COMPOUND HETEROGENEOUS HOMOGENEOUS Uniform or evenly mixed throughout (a solution)

Double Bubble Mind Map Alike Different Topic

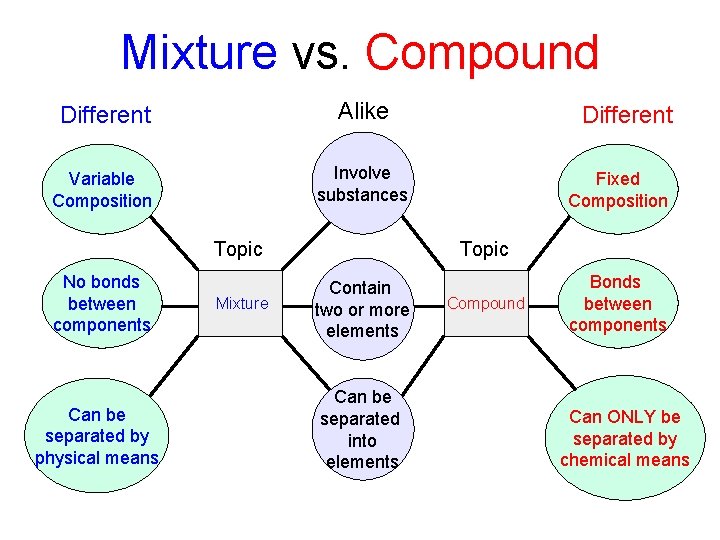
Mixture vs. Compound Different Alike Variable Composition Involve substances Topic No bonds between components Can be separated by physical means Mixture Different Fixed Composition Topic Contain two or more elements Can be separated into elements Compound Bonds between components Can ONLY be separated by chemical means

Contrast… 24 K GOLD 24/24 atoms are gold pure gold element Au 14 K GOLD 14/24 atoms are gold mixture of gold & other metals homogeneous mixture e. g. , Au + Cu

Compound Composition All samples of a given compound Always have the same composition Every sample of Na. Cl tastes the same, melts at the same temp. , and is 39. 3% Na and 60. 7% Cl by mass.

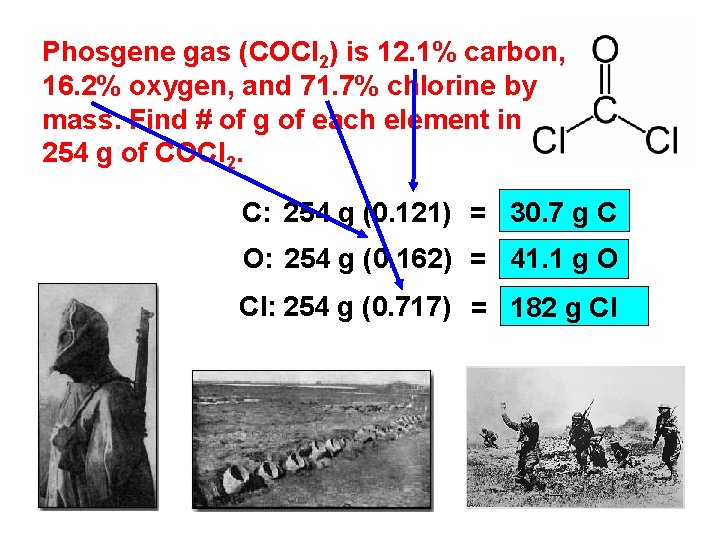
Phosgene gas (COCl 2) is 12. 1% carbon, 16. 2% oxygen, and 71. 7% chlorine by mass. Find # of g of each element in 254 g of COCl 2. C: 254 g (0. 121) = 30. 7 g C O: 254 g (0. 162) = 41. 1 g O Cl: 254 g (0. 717) = 182 g Cl

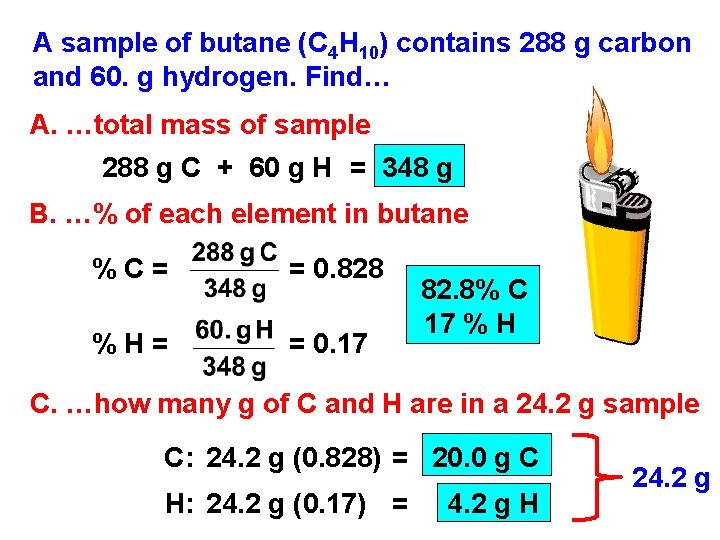
A sample of butane (C 4 H 10) contains 288 g carbon and 60. g hydrogen. Find… A. …total mass of sample 288 g C + 60 g H = 348 g B. …% of each element in butane %C= = 0. 828 %H= = 0. 17 82. 8% C 17 % H C. …how many g of C and H are in a 24. 2 g sample C: 24. 2 g (0. 828) = 20. 0 g C H: 24. 2 g (0. 17) = 4. 2 g H 24. 2 g

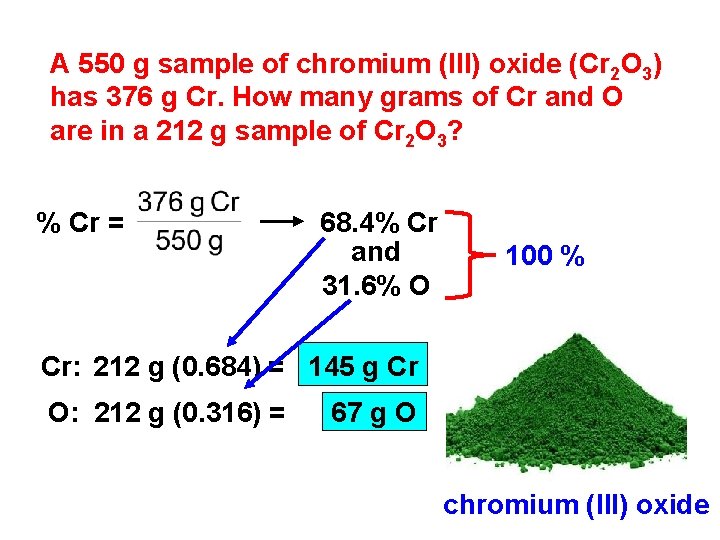
A 550 g sample of chromium (III) oxide (Cr 2 O 3) has 376 g Cr. How many grams of Cr and O are in a 212 g sample of Cr 2 O 3? % Cr = 68. 4% Cr and 31. 6% O 100 % Cr: 212 g (0. 684) = 145 g Cr O: 212 g (0. 316) = 67 g O chromium (III) oxide

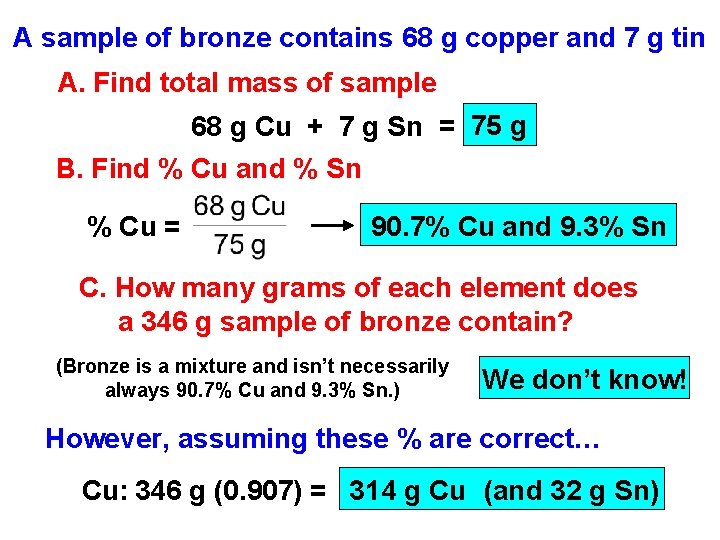
A sample of bronze contains 68 g copper and 7 g tin A. Find total mass of sample 68 g Cu + 7 g Sn = 75 g B. Find % Cu and % Sn % Cu = 90. 7% Cu and 9. 3% Sn C. How many grams of each element does a 346 g sample of bronze contain? (Bronze is a mixture and isn’t necessarily always 90. 7% Cu and 9. 3% Sn. ) We don’t know! However, assuming these % are correct… Cu: 346 g (0. 907) = 314 g Cu (and 32 g Sn)

Separating Mixtures …involves physical means, or physical changes 1. sorting: by color, shape, texture, etc. 2. filter: particle size is different

filtration in the chemistry laboratory

filtration in the “real world”

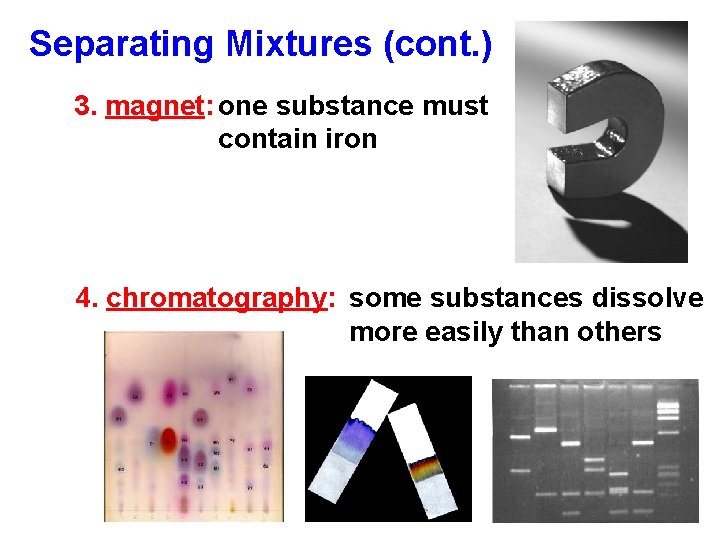
Separating Mixtures (cont. ) 3. magnet: one substance must contain iron 4. chromatography: some substances dissolve more easily than others

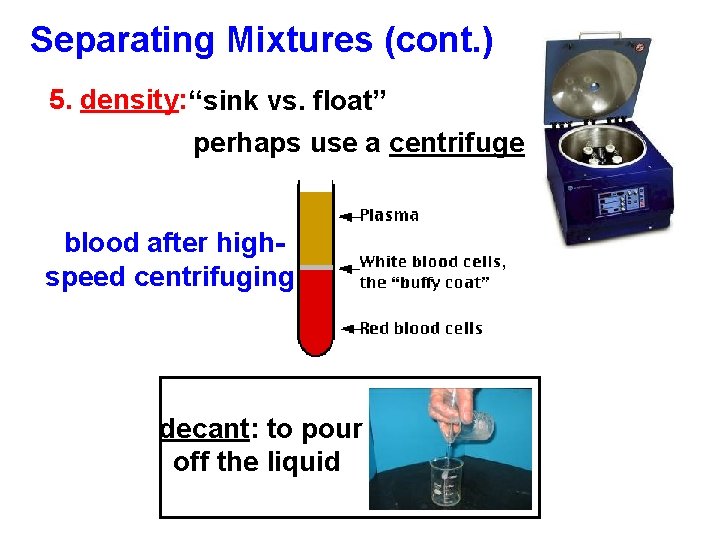
Separating Mixtures (cont. ) 5. density: “sink vs. float” perhaps use a centrifuge blood after highspeed centrifuging decant: to pour off the liquid

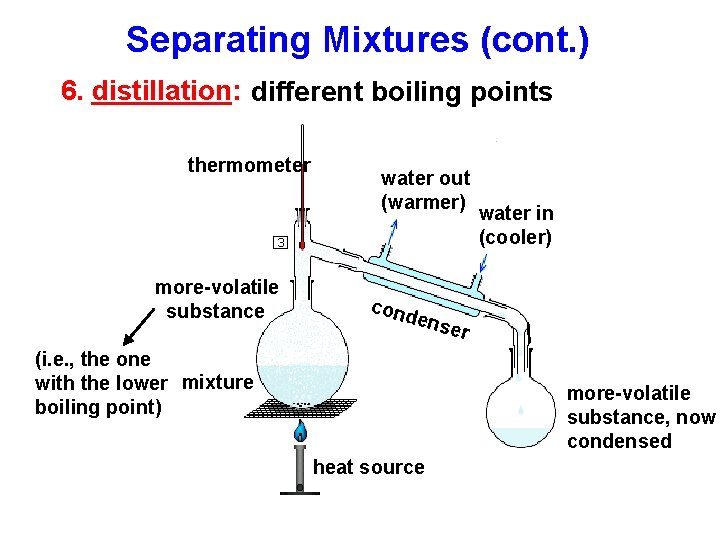
Separating Mixtures (cont. ) 6. distillation: different boiling points thermometer more-volatile substance water out (warmer) cond ense (i. e. , the one with the lower mixture boiling point) water in (cooler) r more-volatile substance, now condensed heat source

No chemical reactions are needed to separate mixtures; substances are NOT bonded dental amalgam

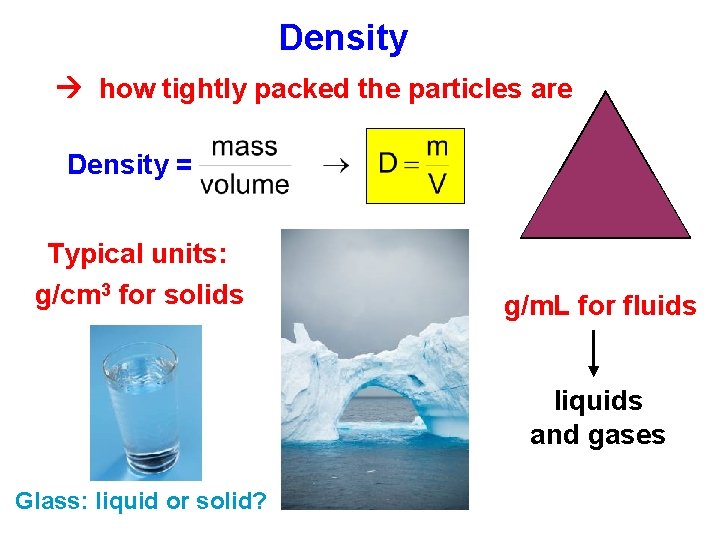
Density how tightly packed the particles are m Density = D Typical units: g/cm 3 for solids V g/m. L for fluids liquids and gases Glass: liquid or solid?

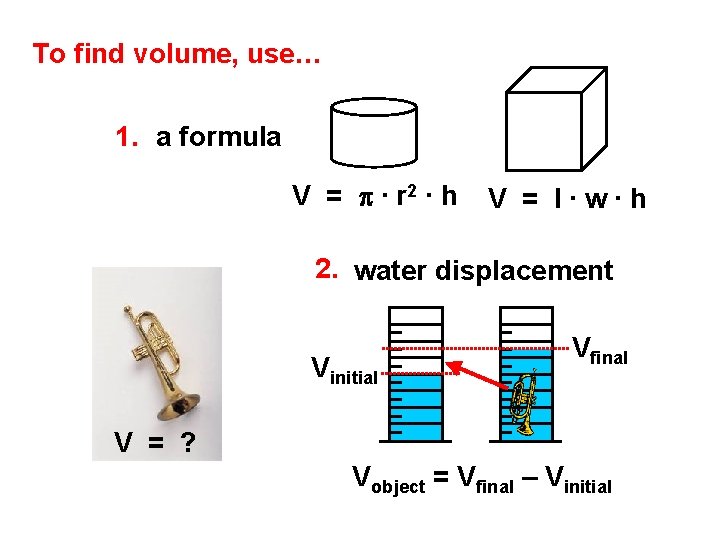
To find volume, use… 1. a formula V = p ∙ r 2 ∙ h V = l∙w∙h 2. water displacement Vinitial Vfinal V = ? Vobject = Vfinal – Vinitial

** Density of water = 1. 0 g/m. L = 1. 0 g/cm 3 Things that are “less dense” float in things that are “more dense. ” (And things that are “more dense” sink in things that are “less dense. ” D < 1 g/cm 3 D > 1 g/cm 3 D < 1 g/cm 3 The density of a liquid or solid is nearly constant, no matter what the sample’s temperature Density of gases is highly dependent on temperature

Ironwood Trees • Several different varieties of hardwood trees, having densities between 1. 34 and 1. 49 g/cm 3 • Most dense species is South African Ironwood (black ironwood) • Olea laurifolia • Found in Florida and West Indies • D = 1. 49 g/cm 3

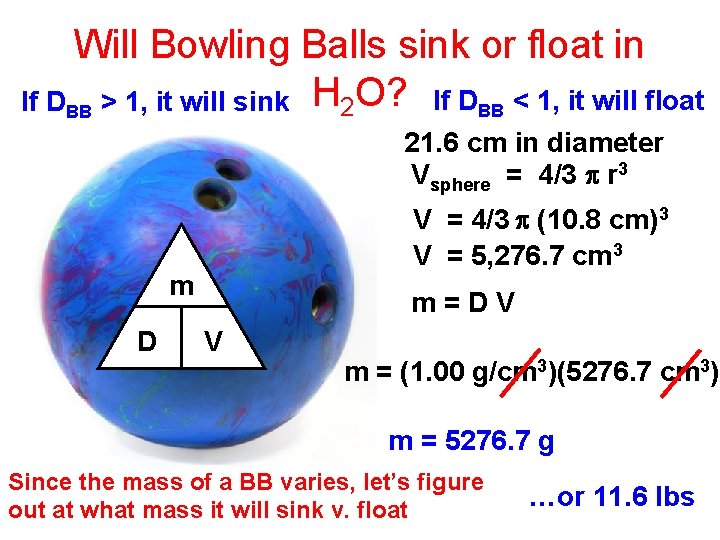
Will Bowling Balls sink or float in If DBB > 1, it will sink H 2 O? If DBB < 1, it will float 21. 6 cm in diameter Vsphere = 4/3 p r 3 V = 4/3 p (10. 8 cm)3 V = 5, 276. 7 cm 3 m D m=DV V m = (1. 00 g/cm 3)(5276. 7 cm 3) m = 5276. 7 g Since the mass of a BB varies, let’s figure out at what mass it will sink v. float …or 11. 6 lbs

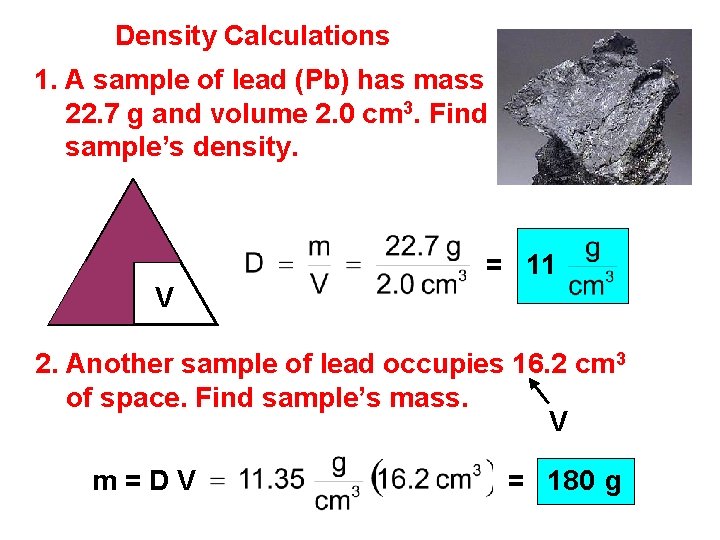
Density Calculations 1. A sample of lead (Pb) has mass 22. 7 g and volume 2. 0 cm 3. Find sample’s density. m D = 11 V 2. Another sample of lead occupies 16. 2 cm 3 of space. Find sample’s mass. V m=DV = 180 g

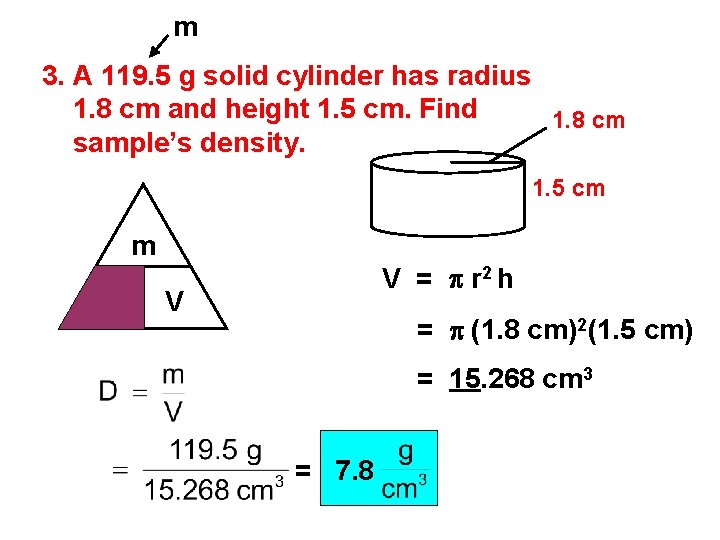
m 3. A 119. 5 g solid cylinder has radius 1. 8 cm and height 1. 5 cm. Find 1. 8 cm sample’s density. 1. 5 cm m D V = p r 2 h V = p (1. 8 cm)2(1. 5 cm) = 15. 268 cm 3 = 7. 8

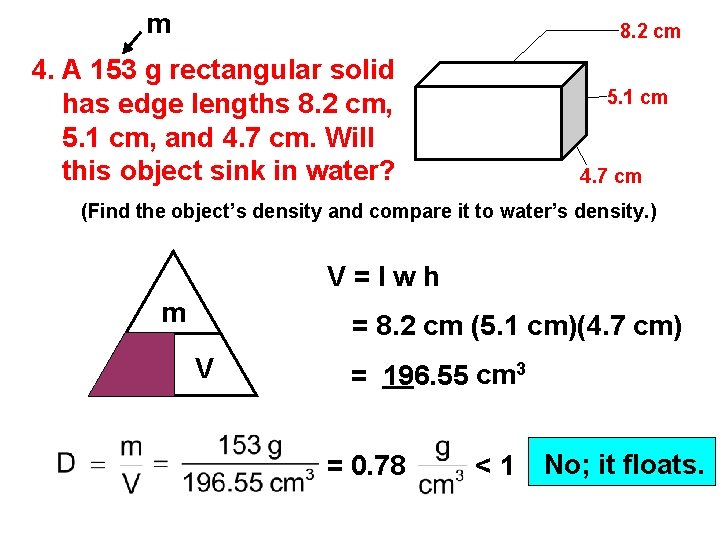
m 8. 2 cm 4. A 153 g rectangular solid has edge lengths 8. 2 cm, 5. 1 cm, and 4. 7 cm. Will this object sink in water? 5. 1 cm 4. 7 cm (Find the object’s density and compare it to water’s density. ) V=lwh m D = 8. 2 cm (5. 1 cm)(4. 7 cm) V = 196. 55 cm 3 = 0. 78 <1 No; it floats.

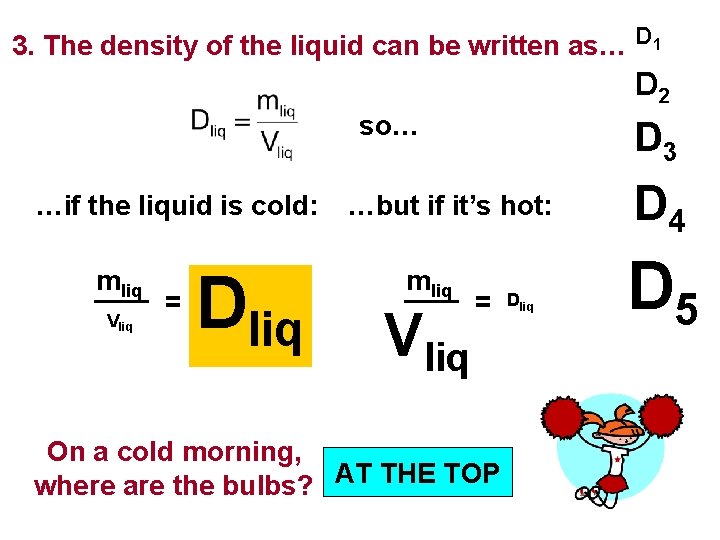
Galilean Thermometer Problem On a cold morning, a teacher walks into a cold classroom and notices that all bulbs in the Galilean thermometer are huddled in a group. Where are the bulbs? At the top of thermometer, at the bottom or elsewhere? D 1 1. Bulbs have essentially fixed masses D 2 D 3 D 4 D 5 and volumes. Therefore, each bulb has a relatively fixed density. 2. The surrounding liquid has a fixed mass, but its volume is extremely temperature-dependent.

3. The density of the liquid can be written as… D 1 D 2 so… …if the liquid is cold: mliq Vliq = Dliq D 3 …but if it’s hot: mliq Vliq = On a cold morning, where are the bulbs? AT THE TOP Dliq D 4 D 5

Osmium •

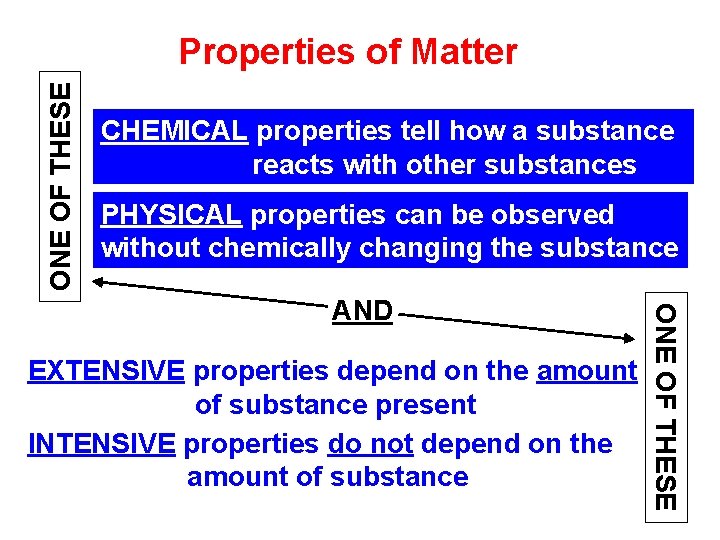
ONE OF THESE Properties of Matter CHEMICAL properties tell how a substance reacts with other substances PHYSICAL properties can be observed without chemically changing the substance EXTENSIVE properties depend on the amount of substance present INTENSIVE properties do not depend on the amount of substance ONE OF THESE AND

Examples: electrical conductivity…………. . … P, I reactivity with water……………. . . C, I heat content (total energy)………………. . … P, E ductile: can be drawn (pulled) into wire…. . P, I malleable: can be hammered into shape… P, I brittle………………………. P, I magnetism…………………… P, I

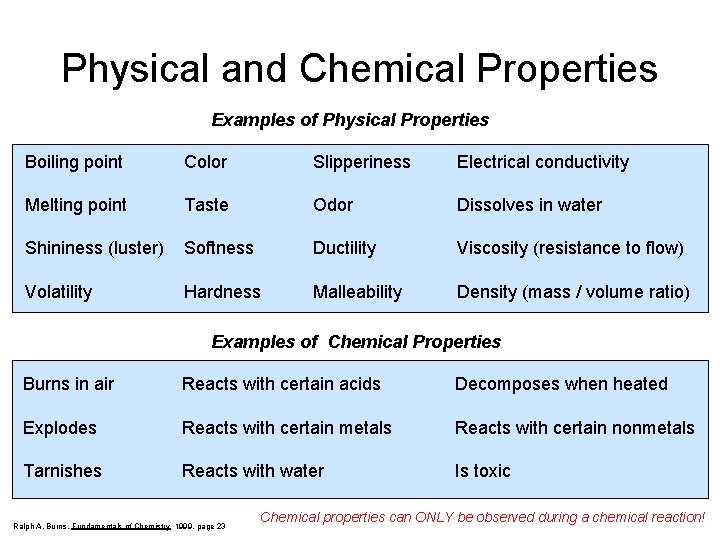
Physical and Chemical Properties Examples of Physical Properties Boiling point Color Slipperiness Electrical conductivity Melting point Taste Odor Dissolves in water Shininess (luster) Softness Ductility Viscosity (resistance to flow) Volatility Hardness Malleability Density (mass / volume ratio) Examples of Chemical Properties Burns in air Reacts with certain acids Decomposes when heated Explodes Reacts with certain metals Reacts with certain nonmetals Tarnishes Reacts with water Is toxic Ralph A. Burns, Fundamentals of Chemistry 1999, page 23 Chemical properties can ONLY be observed during a chemical reaction!

e g n The formation of a a h C l a c i m compound e h C e g n The formation of a a h C l a c i s Phy mixture

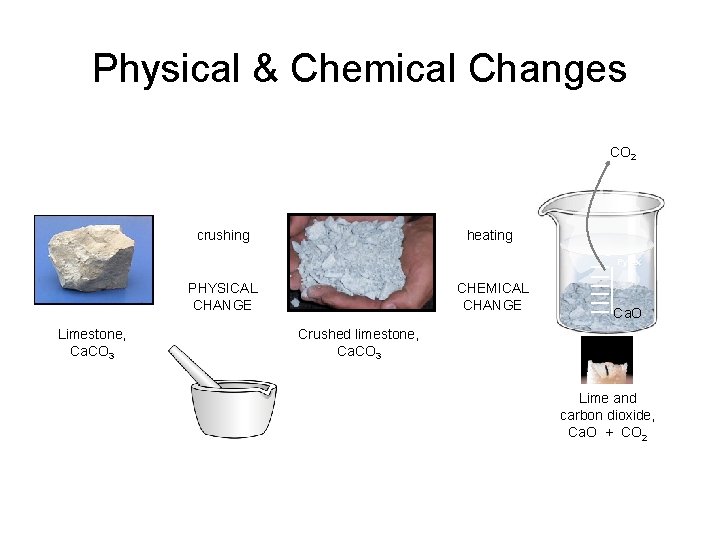
Physical & Chemical Changes CO 2 crushing heating Pyrex PHYSICAL CHANGE Limestone, Ca. CO 3 CHEMICAL CHANGE Ca. O Crushed limestone, Ca. CO 3 Lime and carbon dioxide, Ca. O + CO 2

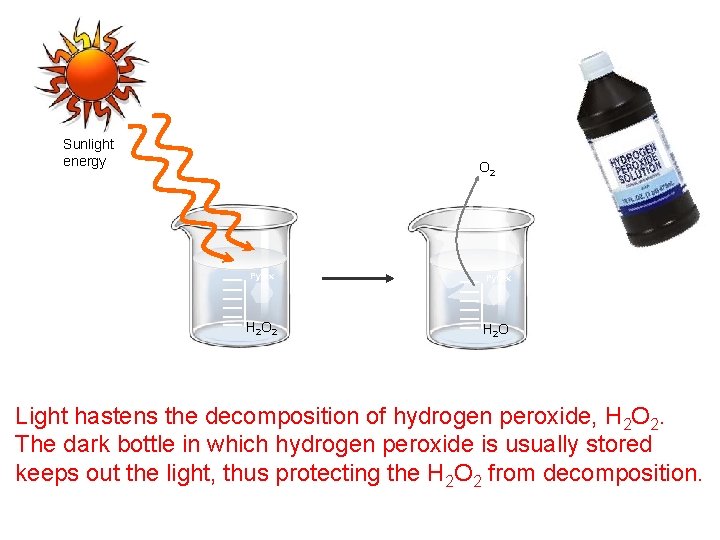
Sunlight energy O 2 Pyrex H 2 O Light hastens the decomposition of hydrogen peroxide, H 2 O 2. The dark bottle in which hydrogen peroxide is usually stored keeps out the light, thus protecting the H 2 O 2 from decomposition.

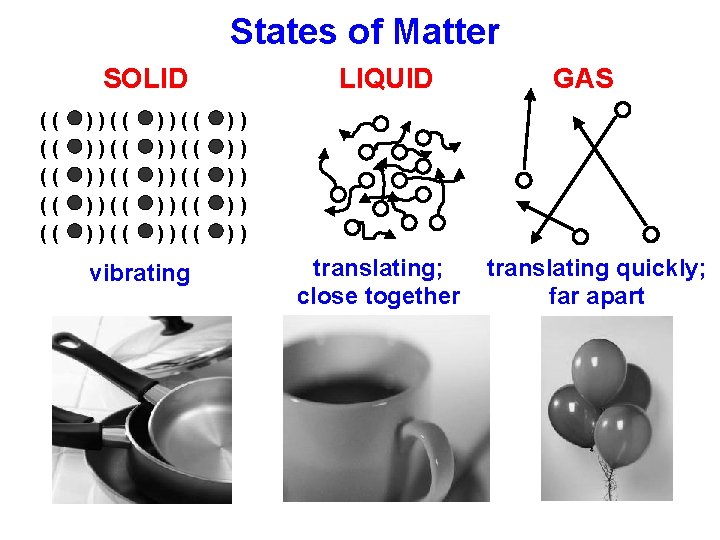
States of Matter SOLID (( (( (( ))(( ))(( ))(( vibrating LIQUID GAS )) )) )) translating; close together translating quickly; far apart

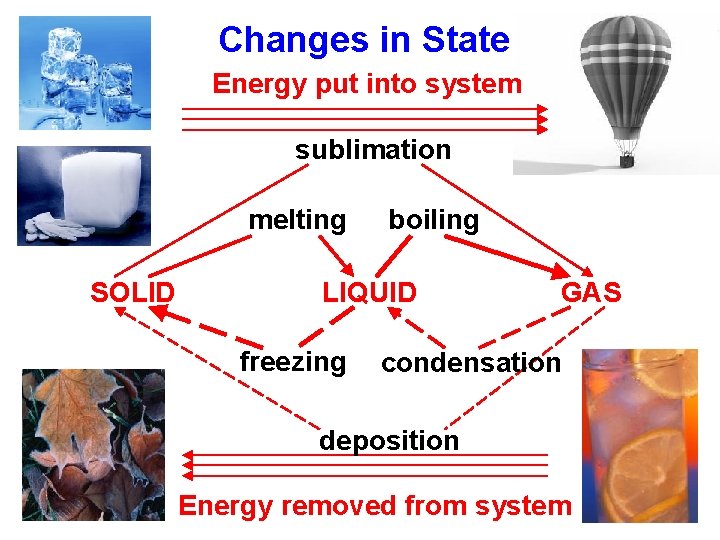
Changes in State Energy put into system sublimation melting SOLID boiling LIQUID freezing GAS condensation deposition Energy removed from system

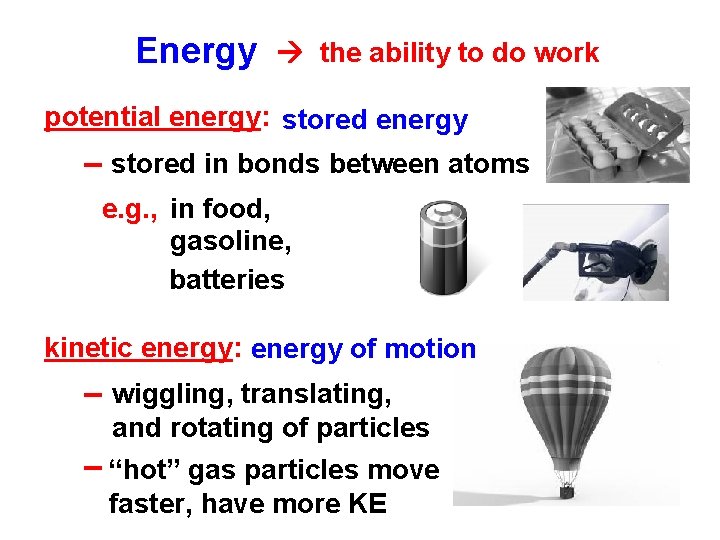
Energy the ability to do work potential energy: stored energy -- stored in bonds between atoms e. g. , in food, gasoline, batteries kinetic energy: energy of motion -- wiggling, translating, and rotating of particles -- “hot” gas particles move faster, have more KE

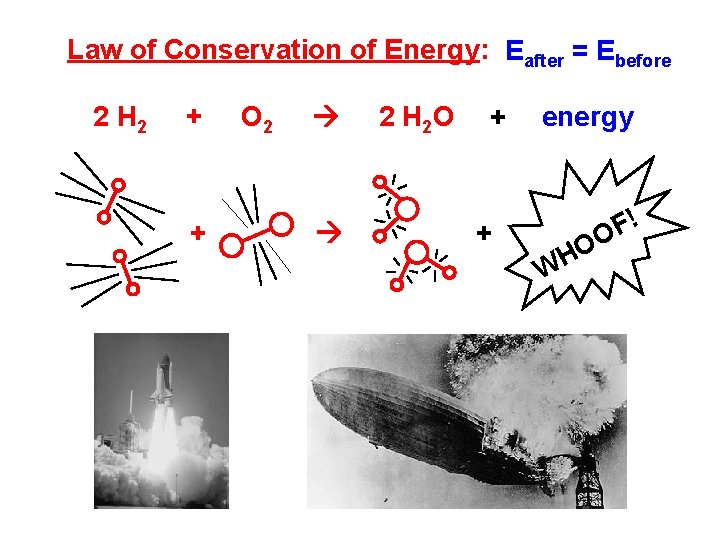
Law of Conservation of Energy: Eafter = Ebefore 2 H 2 + + O 2 2 H 2 O + + energy O H W ! F O

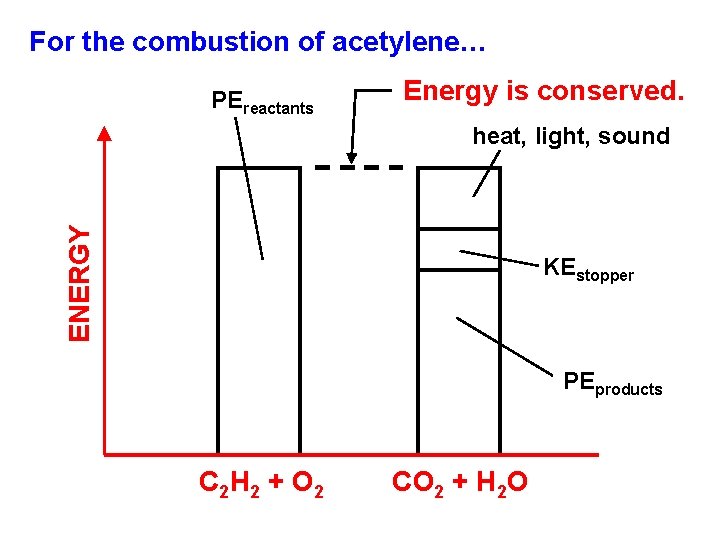
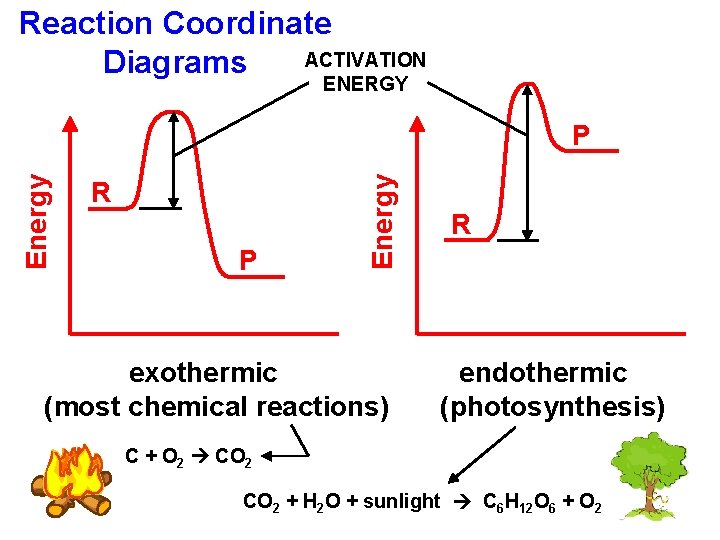
For the combustion of acetylene… PEreactants Energy is conserved. ENERGY heat, light, sound KEstopper PEproducts C 2 H 2 + O 2 CO 2 + H 2 O

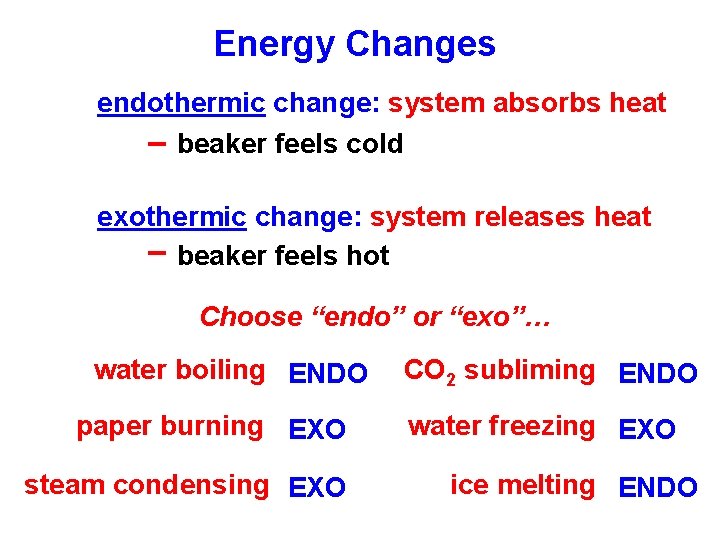
Energy Changes endothermic change: system absorbs heat -- beaker feels cold exothermic change: system releases heat -- beaker feels hot Choose “endo” or “exo”… water boiling ENDO paper burning EXO steam condensing EXO CO 2 subliming ENDO water freezing EXO ice melting ENDO

Reaction Coordinate ACTIVATION Diagrams ENERGY R P Energy P exothermic (most chemical reactions) R endothermic (photosynthesis) C + O 2 CO 2 + H 2 O + sunlight C 6 H 12 O 6 + O 2

The Mole The mole is the SI unit for “amount of substance. ” Atoms are so small, it is impossible to count them by the dozens, thousands, or even millions. To count atoms, we use the concept of the mole 1 mole of atoms = 602, 000, 000, 000 atoms 6. 02 x 1023 atoms That is, 1 mole of atoms = _____

How Big is a Mole? …about the size of a chipmunk, weighing about 5 oz. (140 g), and having a length of about 7 inches (18 cm). I Meant, “How Big is 6. 02 x 1023? ” BIG. 6. 02 x 1023 marbles would cover the entire Earth (including the oceans) …to a height of 2 miles. 6. 02 x 1023 $1 bills stacked face-to-face would stretch from the Sun to Pluto …and back … 7. 5 million times. (It takes light 9, 500 years to travel that far)

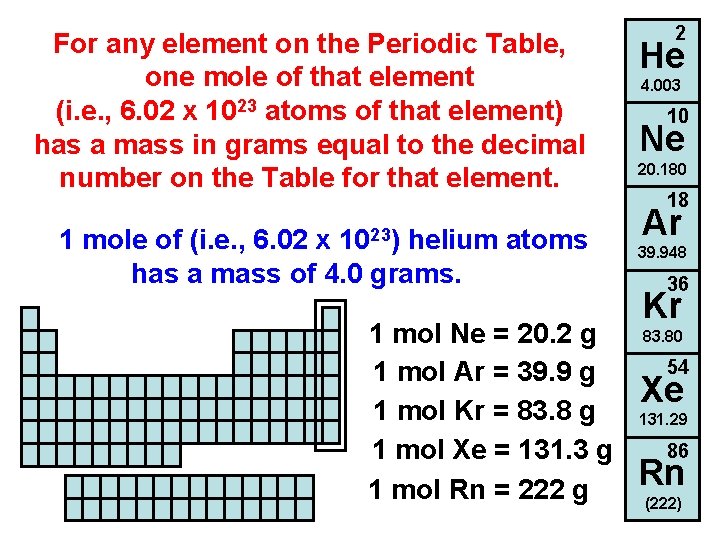
For any element on the Periodic Table, one mole of that element (i. e. , 6. 02 x 1023 atoms of that element) has a mass in grams equal to the decimal number on the Table for that element. 1023) 1 mole of (i. e. , 6. 02 x helium atoms has a mass of 4. 0 grams. 1 mol Ne = 20. 2 g 1 mol Ar = 39. 9 g 1 mol Kr = 83. 8 g 1 mol Xe = 131. 3 g 1 mol Rn = 222 g 2 He 4. 003 10 Ne 20. 180 18 Ar 39. 948 36 Kr 83. 80 54 Xe 131. 29 86 Rn (222)

)g selcitrap 32

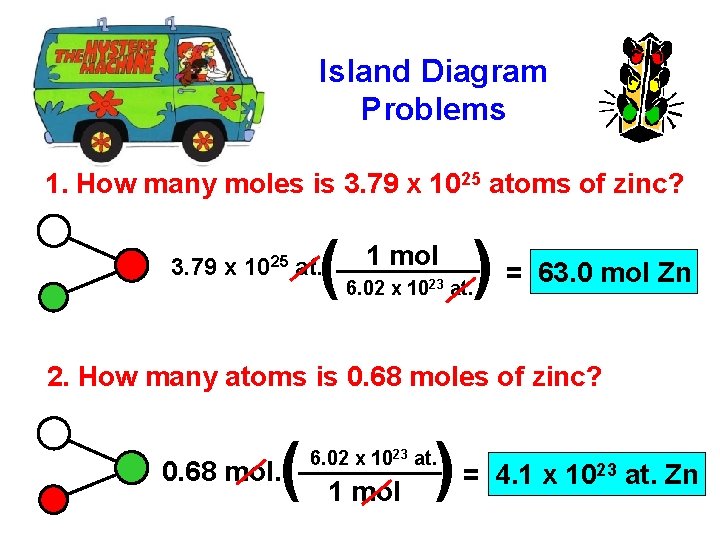
Island Diagram Problems 1. How many moles is 3. 79 x 1025 atoms of zinc? ( 3. 79 x 1025 at. 1 mol ) = 63. 0 mol Zn 6. 02 x 1023 at. 2. How many atoms is 0. 68 moles of zinc? ( 0. 68 mol. ) = 4. 1 x 10 6. 02 x 1023 at. 1 mol 23 at. Zn

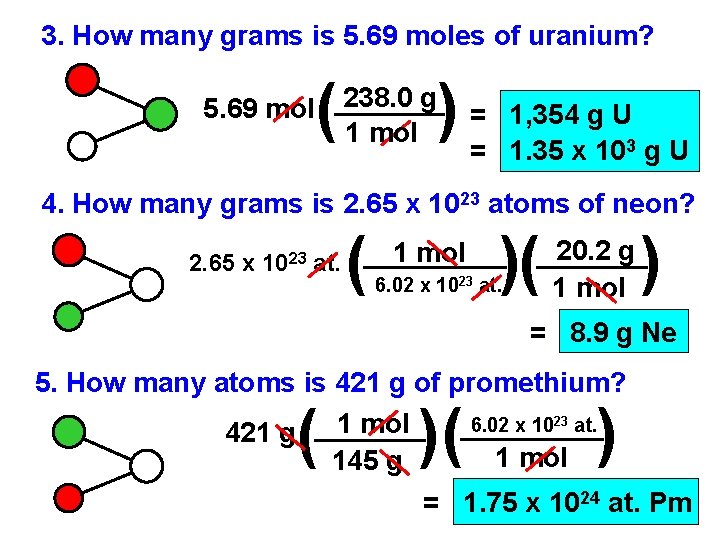
3. How many grams is 5. 69 moles of uranium? ( ) == 5. 69 mol 238. 0 g 1 mol 1, 354 g U 1. 35 x 103 g U 4. How many grams is 2. 65 x 1023 atoms of neon? 2. 65 x 1023 at. ( 1 mol )( 6. 02 x 1023 at. 20. 2 g 1 mol ) = 8. 9 g Ne 5. How many atoms is 421 g of promethium? 6. 02 x 1023 at. 1 mol 421 g 1 mol 145 g ( )( ) = 1. 75 x 1024 at. Pm
 What is the answer
What is the answer Kuei honors chemistry
Kuei honors chemistry Honors chemistry summer assignment
Honors chemistry summer assignment Bond order
Bond order Flow of energy vs flow of matter
Flow of energy vs flow of matter Honors biology unit 4 test
Honors biology unit 4 test Used so often as to lack freshness or originality
Used so often as to lack freshness or originality Unit 2 matter and energy
Unit 2 matter and energy Grey vs white matter
Grey vs white matter Primary taste cortex
Primary taste cortex Gray matter and white matter
Gray matter and white matter What is gray matter in the brain
What is gray matter in the brain Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Definition of substance
Definition of substance Chemistry matter and change chapter 7
Chemistry matter and change chapter 7 Chapter 10 study guide the mole
Chapter 10 study guide the mole Chemistry matter and change chapter 7
Chemistry matter and change chapter 7 Chemistry matter and change chapter 6
Chemistry matter and change chapter 6 Chemistry matter and change chapter 10
Chemistry matter and change chapter 10 2 matter and change answer key
2 matter and change answer key Deped order no. 36 s. 2016
Deped order no. 36 s. 2016 Ucsb frap
Ucsb frap Honors geometry parallel lines and transversals worksheet
Honors geometry parallel lines and transversals worksheet Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Section 1 composition of matter
Section 1 composition of matter Chapter 2 section 1 classifying matter answers
Chapter 2 section 1 classifying matter answers Section 1 composition of matter chapter 15 answer key
Section 1 composition of matter chapter 15 answer key Examples of matter in chemistry
Examples of matter in chemistry Matter flowchart
Matter flowchart Matter flowchart chemistry
Matter flowchart chemistry Graphic organizer matter
Graphic organizer matter Chapter 4 basic food chemistry the nature of matter
Chapter 4 basic food chemistry the nature of matter Chapter 4 basic food chemistry the nature of matter
Chapter 4 basic food chemistry the nature of matter Chapter 4 basic food chemistry the nature of matter
Chapter 4 basic food chemistry the nature of matter Guiding principles for teaching and learning in mtb-mle
Guiding principles for teaching and learning in mtb-mle Guiding play and puppetry experiences
Guiding play and puppetry experiences Mission vision guiding principles navy
Mission vision guiding principles navy Bias through selection and omission guiding questions
Bias through selection and omission guiding questions Creighton university honors program
Creighton university honors program James scholar honors program
James scholar honors program Honors physics semester 1 review
Honors physics semester 1 review Honors biology ecology test
Honors biology ecology test Economics 4.05 uncle sam's toolbox honors
Economics 4.05 uncle sam's toolbox honors Quadrilateral test review
Quadrilateral test review Honors earth science
Honors earth science Honors math 3
Honors math 3 Hilton honors military program
Hilton honors military program Honors project
Honors project Honors biology properties of water lab
Honors biology properties of water lab Tulane honors program
Tulane honors program Second quarter exam
Second quarter exam Pre calculus chapter 1
Pre calculus chapter 1 Vyi physics
Vyi physics Smc scholars classes
Smc scholars classes Math 3 honors
Math 3 honors Crystalline solid and amorphous solid
Crystalline solid and amorphous solid Algebra 1 honors
Algebra 1 honors Mahurin honors college
Mahurin honors college Honors algebra 2 final exam
Honors algebra 2 final exam Umes honors program
Umes honors program Latin honors smith college
Latin honors smith college Pathfinder shooting star
Pathfinder shooting star Physics honors notes
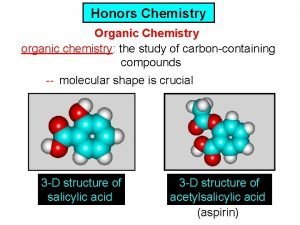
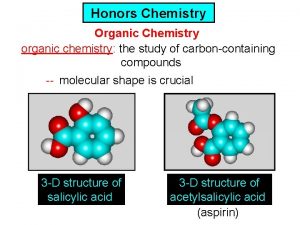
Physics honors notes Ib organic chemistry functional groups
Ib organic chemistry functional groups Organic vs inorganic chemistry
Organic vs inorganic chemistry Phosphorus cycle
Phosphorus cycle Which reverses the normal flow of thermal energy
Which reverses the normal flow of thermal energy