Matter and Change Honors Chemistry Chemistry is a









- Slides: 13

Matter and Change Honors Chemistry

Chemistry is a Physical Science • Chemistry is the study of the composition, structure, and properties of matter and the changes it undergoes. • Branches of Chemistry – – – Organic Chemistry Inorganic Chemistry Physical Chemistry Analytical Chemistry Biochemistry Theoretical Chemistry

Matter and Its Properties • A chemical is any substance that has definite composition. • Matter is anything that has mass and volume. – Mass is the amount of matter in an object. – Volume is how much space the object occupies.

Building Blocks of Matter • An atom is the smallest unit of matter that maintains the properties of the element. • An element is a pure substance made of only one kind of atom. • A compound is a substance made of the atoms of two or more elements that are chemically bonded.

Properties of Matter • Extensive properties depend on how much matter is present. – i. e. mass, volume, etc. • Intensive properties do not depend upon the amount of matter. – Boiling point, melting point, density, etc.

Physical Properties and Physical Changes • Physical properties can be observed without changing the identity of the substance. – i. e. color, mass, odor, boiling point, density. • Physical changes do not change the identity of the substance. – i. e. phase changes, solutes dissolving, cutting, tearing, etc.

Chemical Properties and Chemical Changes • Chemical properties can only be observed when a chemical reaction has occurred. • Chemical changes occur when matter undergoes a chemical reaction and new substances are formed.

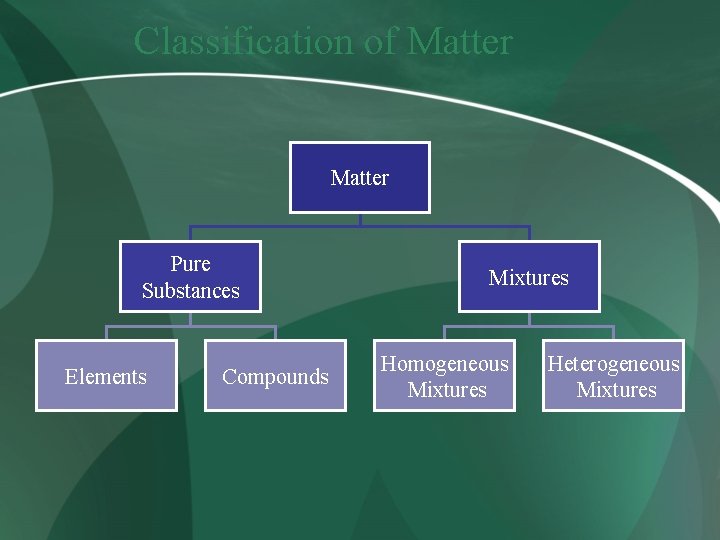
Classification of Matter Pure Substances Elements Compounds Mixtures Homogeneous Mixtures Heterogeneous Mixtures

Mixtures • Homogeneous mixtures include solutions and alloys. • In a homogeneous mixture, the particles are not observable. • Heterogeneous mixtures occur whenever we can see the particles that have been mixed together.

Pure Substances • Elements • Compounds

Introduction to the Periodic Table • The vertical columns are called groups or families. There are 18 of them. • The horizontal rows are called periods, there are 7 of them.

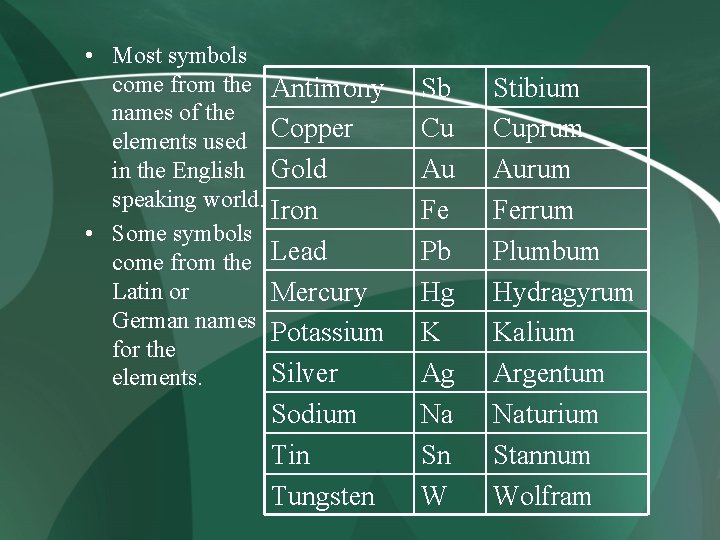
• Most symbols come from the Antimony names of the elements used Copper in the English Gold speaking world. Iron • Some symbols come from the Lead Latin or Mercury German names Potassium for the Silver elements. Sodium Tin Tungsten Sb Cu Au Fe Pb Hg K Ag Na Sn W Stibium Cuprum Aurum Ferrum Plumbum Hydragyrum Kalium Argentum Naturium Stannum Wolfram

Types of Elements • Metals • Non-Metals • Semi-Metals
 Chemistry matter and change chapter 7
Chemistry matter and change chapter 7 Chapter 10 study guide the mole
Chapter 10 study guide the mole Chemistry matter and change chapter 7
Chemistry matter and change chapter 7 Chemistry matter and change chapter 6
Chemistry matter and change chapter 6 Chemistry matter and change chapter 10 the mole answer key
Chemistry matter and change chapter 10 the mole answer key Chemistry matter and change answer key chapter 2
Chemistry matter and change answer key chapter 2 Kuei honors chemistry
Kuei honors chemistry Chemistry unit 4 review answers
Chemistry unit 4 review answers Honors chemistry summer assignment
Honors chemistry summer assignment Bond order formula
Bond order formula Whats gray matter
Whats gray matter Gyrus and sulcus function
Gyrus and sulcus function Gray matter and white matter
Gray matter and white matter What is grey and white matter
What is grey and white matter