Types of Mixtures Solutions Solutions are homogeneous mixtures










- Slides: 13

Types of Mixtures

Solutions • Solutions are homogeneous mixtures made up of two components. • The part of the solution that does the dissolving is called the solvent. • The part of the solution that gets dissolved is called the solute. • In a sugar water solution, what is the solute? • Sugar • In a sugar water solution, what is the solvent? • Water

Solutions (continued) The solute particles are < 1 nm in diameter. How large are these particles in cm? 0. 0000001 cm As a result, solutions often appear clear. Due to the extremely small particle size solutions remain mixed and do not separate (settle out) upon standing. • Solutions cannot be separated by filtration (particles are so small they pass through the paper with the solvent. • Examples include: kool-aid, flat soft drink • • •

Solutions

Colloids • Colloids are heterogeneous mixtures. • The particles mixed into the main component are between 1 and 100 nm in diameter. • Due to the slightly larger particle size, colloids appear cloudy. • Although the particles are larger than in a solution, they are still small enough that they stay mixed and do not separate upon standing and cannot be separated by filtration. • Examples: milk, whipped cream

Colloids

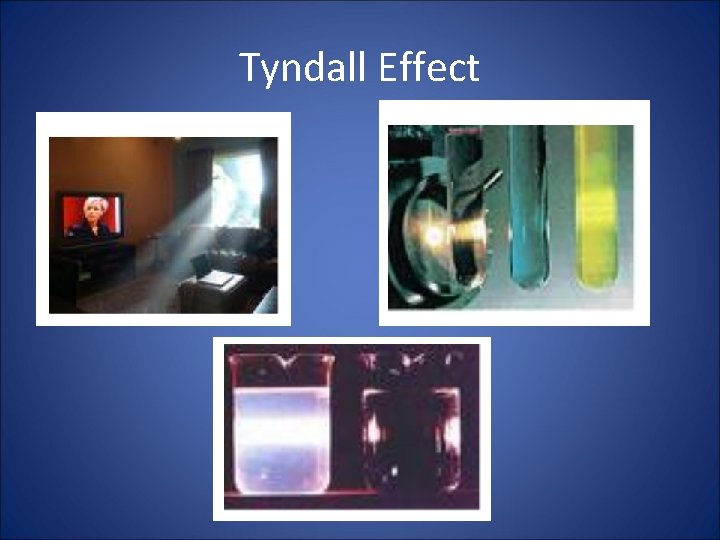
Tyndall Effect • Particles in a colloid are large enough that they will reflect (scatter) light. • This phenomenon is referred to as the Tyndall Effect.

Tyndall Effect

Suspensions • Suspensions are also heterogeneous mixtures. • The particles in a suspension are the largest and have a diameter > 100 nm. • Due to the larger size these particles will separate (settle out) upon standing. • If all of the particles have separated, the liquid may appear clear. If not, the liquid may appear cloudy.

Suspensions • The larger particle size allows for suspensions to be separated by filtration. • The Tyndall effect may be observed if the particles have not settled out. • Examples: muddy water, Italian salad dressing.

Suspension

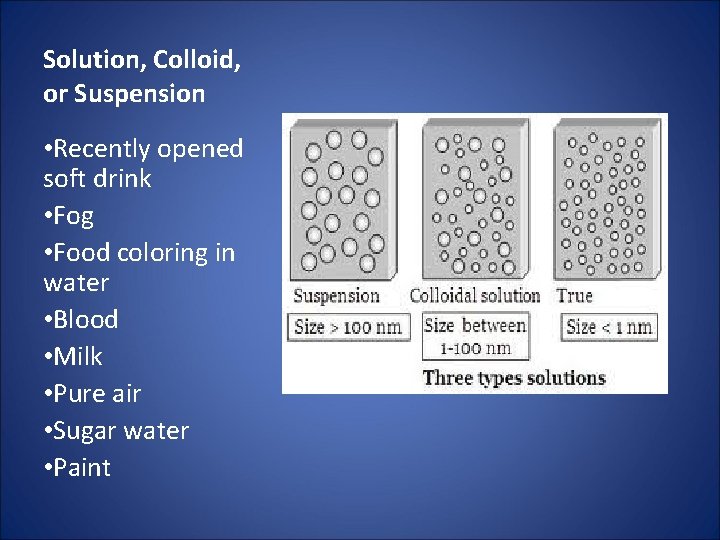
Solution, Colloid, or Suspension • Recently opened soft drink • Fog • Food coloring in water • Blood • Milk • Pure air • Sugar water • Paint

 Antigentest åre
Antigentest åre Are all solutions homogeneous mixtures
Are all solutions homogeneous mixtures Are all aqueous solutions homogeneous
Are all aqueous solutions homogeneous Are all aqueous solutions homogeneous
Are all aqueous solutions homogeneous Solutions are homogeneous mixtures
Solutions are homogeneous mixtures Application of homogeneous differential equation
Application of homogeneous differential equation Elements and compounds examples
Elements and compounds examples Homogeneous mixtures examples
Homogeneous mixtures examples Common homogeneous mixtures
Common homogeneous mixtures Facts about homogeneous mixtures
Facts about homogeneous mixtures Are solutions homogeneous
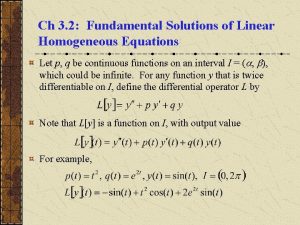
Are solutions homogeneous Fundamental solutions of linear homogeneous equations
Fundamental solutions of linear homogeneous equations Gas and liquid solution example
Gas and liquid solution example Homogeneous mixture and solution
Homogeneous mixture and solution