Separation Process Separation Methods Separation Methods 1 Separation





- Slides: 63

Separation Process

Separation Methods

Separation Methods 1. Separation Methods The separation of chemical mixtures into their constituents has been practiced for millennia. Perfume Metal Liquor Salt

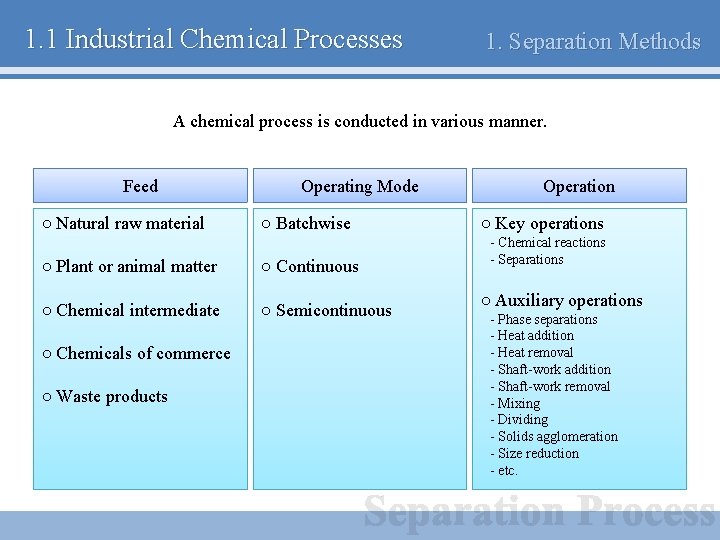
1. 1 Industrial Chemical Processes 1. Separation Methods A chemical process is conducted in various manner. Feed ○ Natural raw material Operating Mode ○ Batchwise ○ Plant or animal matter ○ Continuous ○ Chemical intermediate ○ Semicontinuous ○ Chemicals of commerce ○ Waste products Operation ○ Key operations - Chemical reactions - Separations ○ Auxiliary operations - Phase separations - Heat addition - Heat removal - Shaft-work addition - Shaft-work removal - Mixing - Dividing - Solids agglomeration - Size reduction - etc.

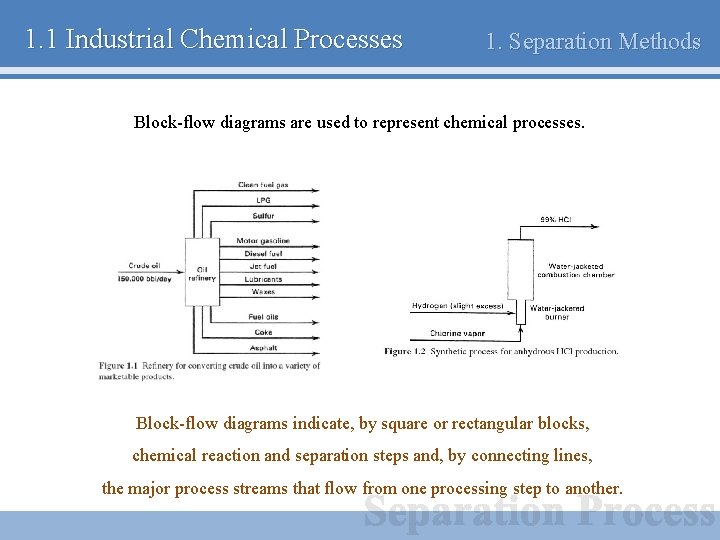
1. 1 Industrial Chemical Processes 1. Separation Methods Block-flow diagrams are used to represent chemical processes. Block-flow diagrams indicate, by square or rectangular blocks, chemical reaction and separation steps and, by connecting lines, the major process streams that flow from one processing step to another.

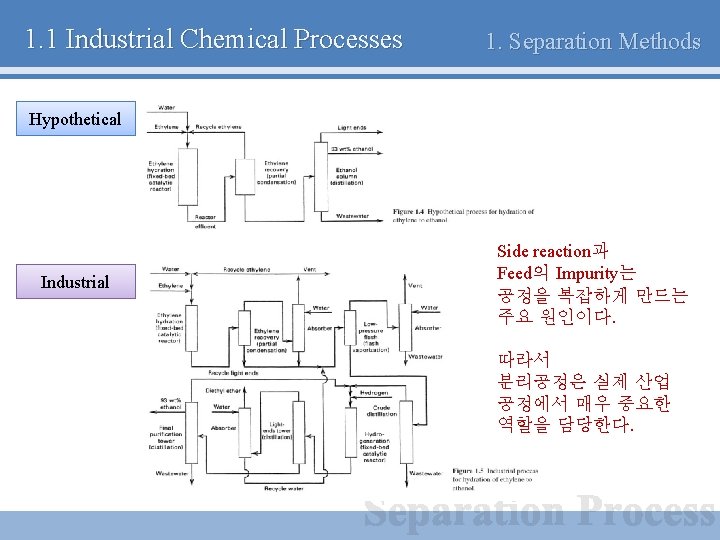
1. 1 Industrial Chemical Processes 1. Separation Methods Hypothetical Industrial Side reaction과 Feed의 Impurity는 공정을 복잡하게 만드는 주요 원인이다. 따라서 분리공정은 실제 산업 공정에서 매우 중요한 역할을 담당한다.

1. 2 Mechanism of Separation 1. Separation Methods Mixing Separation Spontaneous Not spontaneous WHY

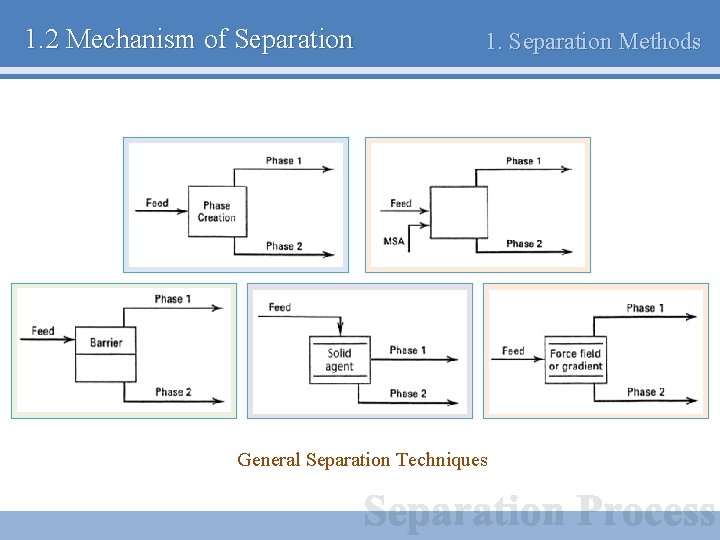
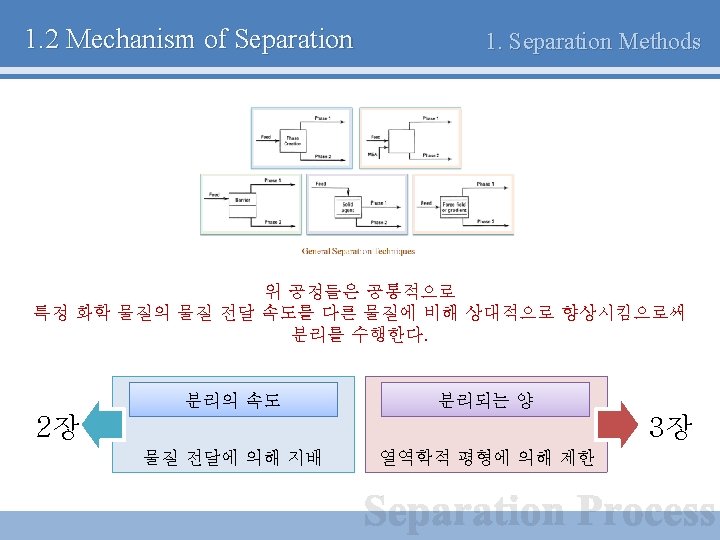
1. 2 Mechanism of Separation 1. Separation Methods General Separation Techniques

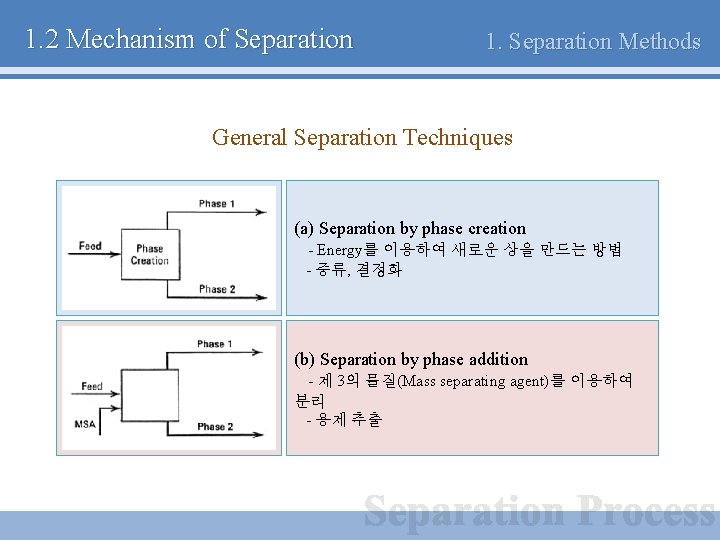
1. 2 Mechanism of Separation 1. Separation Methods General Separation Techniques (a) Separation by phase creation - Energy를 이용하여 새로운 상을 만드는 방법 - 증류, 결정화 (b) Separation by phase addition - 제 3의 물질(Mass separating agent)를 이용하여 분리 - 용제 추출

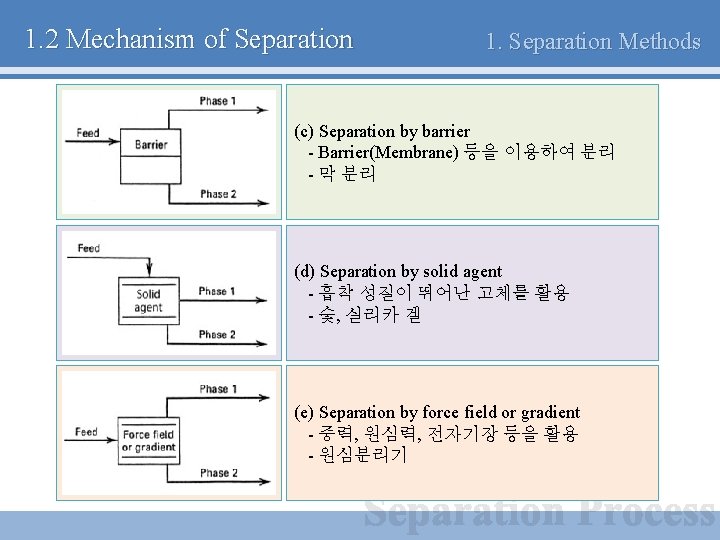
1. 2 Mechanism of Separation 1. Separation Methods (c) Separation by barrier - Barrier(Membrane) 등을 이용하여 분리 - 막 분리 (d) Separation by solid agent - 흡착 성질이 뛰어난 고체를 활용 - 숯, 실리카 겔 (e) Separation by force field or gradient - 중력, 원심력, 전자기장 등을 활용 - 원심분리기


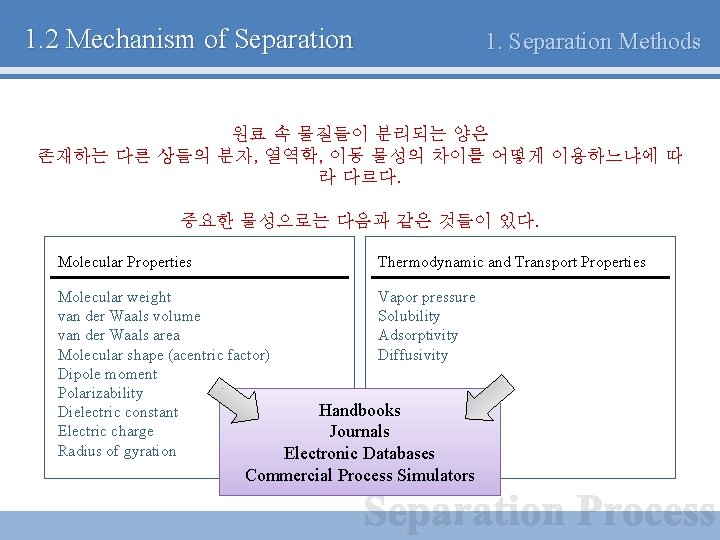
1. 2 Mechanism of Separation 1. Separation Methods 원료 속 물질들이 분리되는 양은 존재하는 다른 상들의 분자, 열역학, 이동 물성의 차이를 어떻게 이용하느냐에 따 라 다르다. 중요한 물성으로는 다음과 같은 것들이 있다. Molecular Properties Thermodynamic and Transport Properties Molecular weight Vapor pressure van der Waals volume Solubility van der Waals area Adsorptivity Molecular shape (acentric factor) Diffusivity Dipole moment Polarizability Handbooks Dielectric constant Electric charge Journals Radius of gyration Electronic Databases Commercial Process Simulators

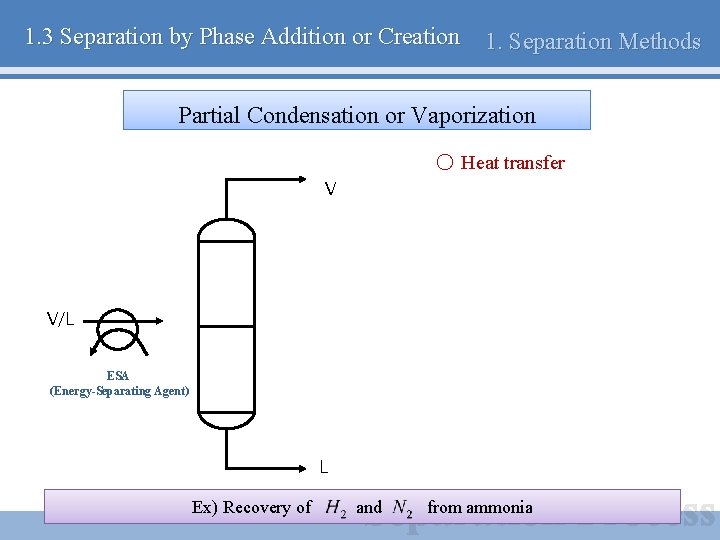
1. 3 Separation by Phase Addition or Creation 1. Separation Methods Partial Condensation or Vaporization ○ Heat transfer V V/L ESA (Energy-Separating Agent) L Ex) Recovery of and from ammonia

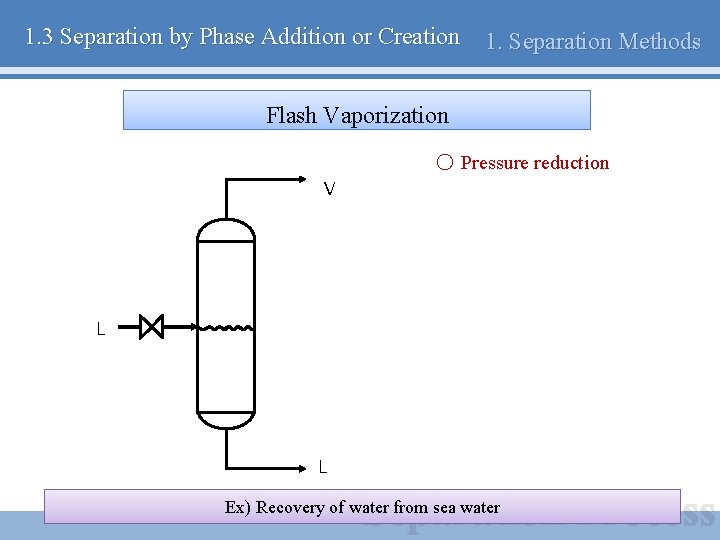
1. 3 Separation by Phase Addition or Creation 1. Separation Methods Flash Vaporization ○ Pressure reduction V L L Ex) Recovery of water from sea water

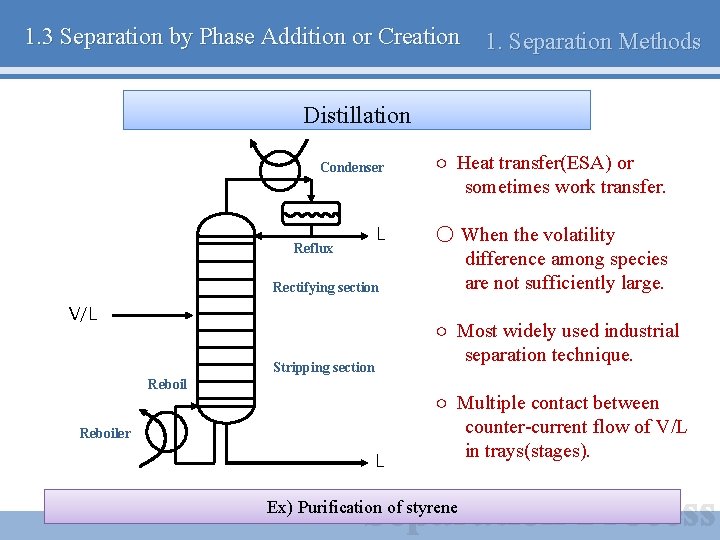
1. 3 Separation by Phase Addition or Creation 1. Separation Methods Distillation Condenser Reflux L Rectifying section V/L ○ Heat transfer(ESA) or sometimes work transfer. ○ When the volatility difference among species are not sufficiently large. ○ Most widely used industrial separation technique. Stripping section Reboiler L ○ Multiple contact between counter-current flow of V/L in trays(stages). Ex) Purification of styrene

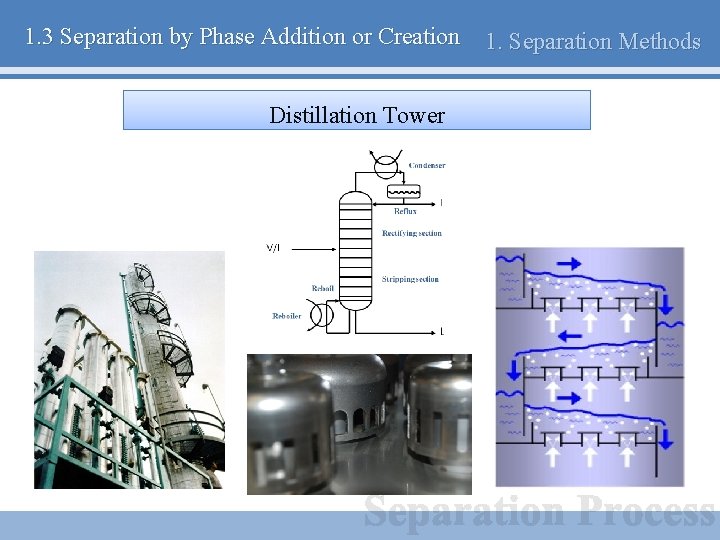
1. 3 Separation by Phase Addition or Creation Distillation Tower 1. Separation Methods

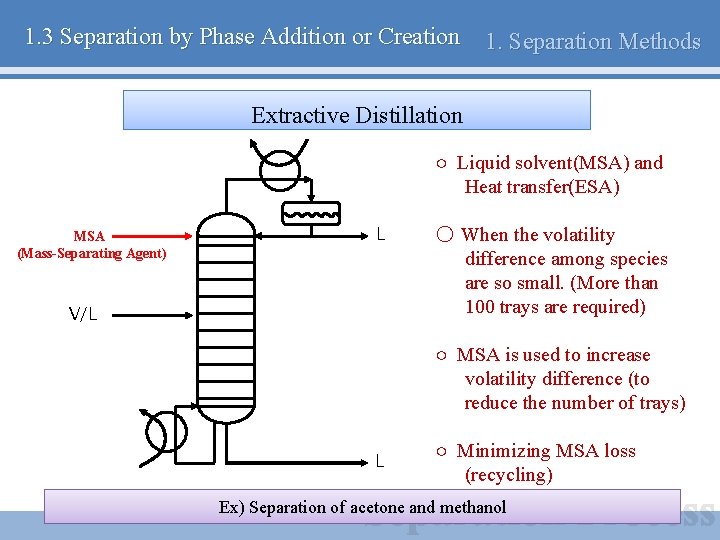
1. 3 Separation by Phase Addition or Creation 1. Separation Methods Extractive Distillation ○ Liquid solvent(MSA) and Heat transfer(ESA) MSA (Mass-Separating Agent) L V/L ○ When the volatility difference among species are so small. (More than 100 trays are required) ○ MSA is used to increase volatility difference (to reduce the number of trays) L ○ Minimizing MSA loss (recycling) Ex) Separation of acetone and methanol

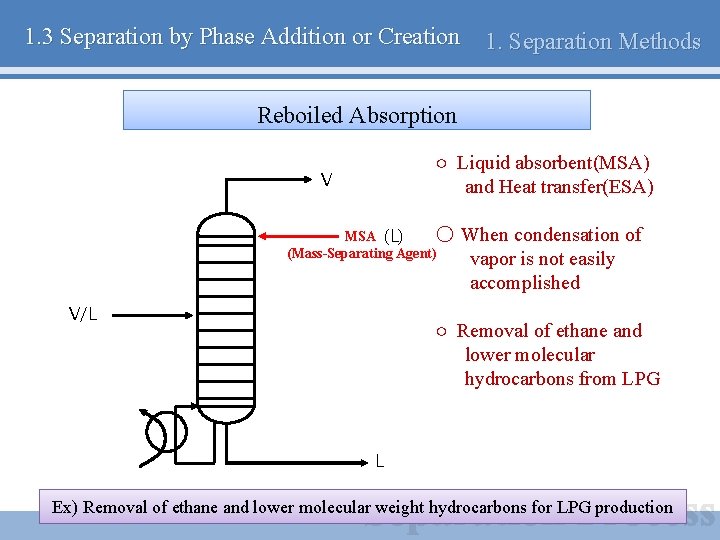
1. 3 Separation by Phase Addition or Creation 1. Separation Methods Reboiled Absorption ○ Liquid absorbent(MSA) and Heat transfer(ESA) V MSA (L) ○ (Mass-Separating Agent) V/L When condensation of vapor is not easily accomplished ○ Removal of ethane and lower molecular hydrocarbons from LPG L Ex) Removal of ethane and lower molecular weight hydrocarbons for LPG production

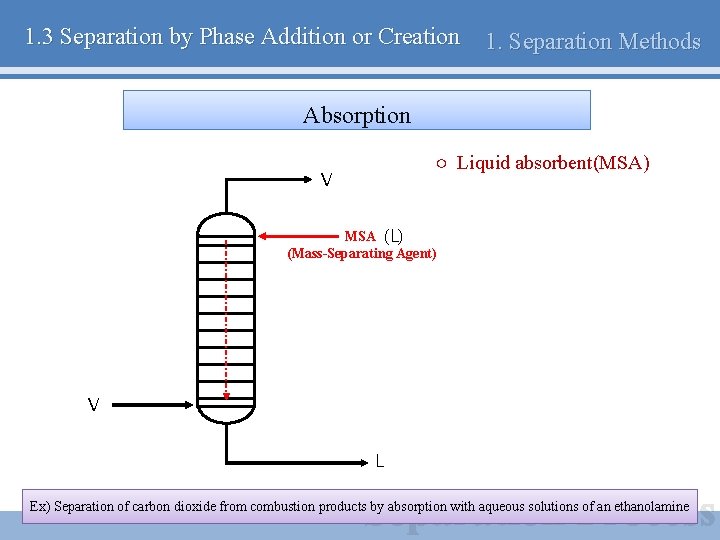
1. 3 Separation by Phase Addition or Creation 1. Separation Methods Absorption ○ Liquid absorbent(MSA) V MSA (L) (Mass-Separating Agent) V L Ex) Separation of carbon dioxide from combustion products by absorption with aqueous solutions of an ethanolamine

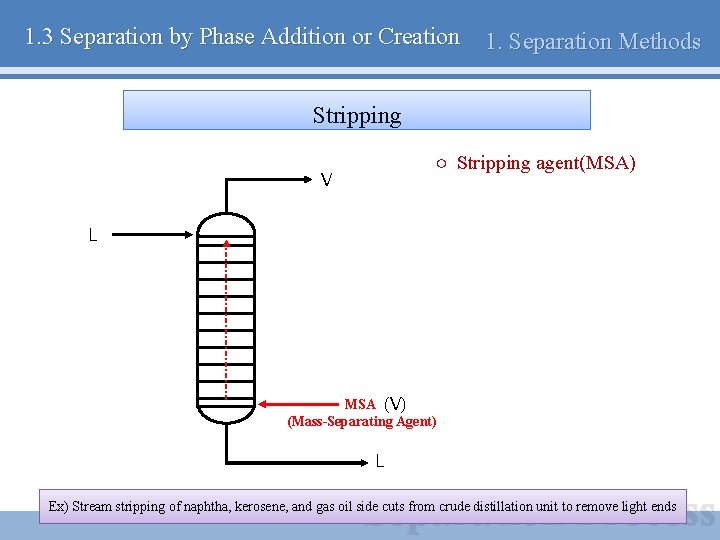
1. 3 Separation by Phase Addition or Creation 1. Separation Methods Stripping ○ Stripping agent(MSA) V L MSA (V) (Mass-Separating Agent) L Ex) Stream stripping of naphtha, kerosene, and gas oil side cuts from crude distillation unit to remove light ends

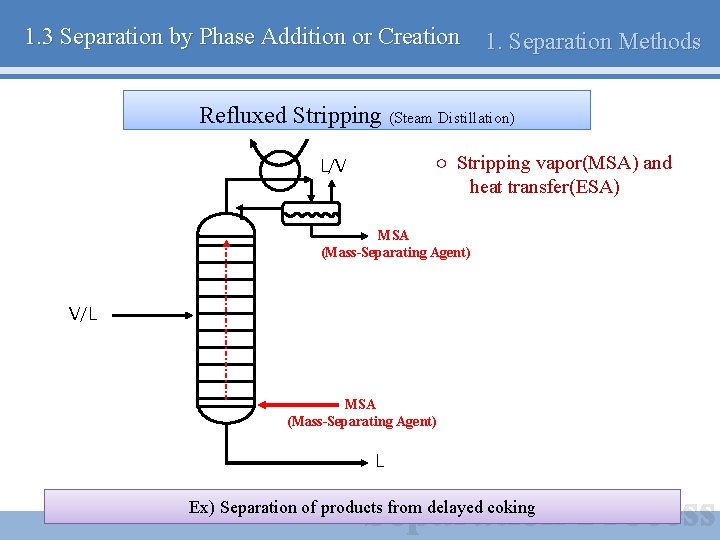
1. 3 Separation by Phase Addition or Creation 1. Separation Methods Refluxed Stripping (Steam Distillation) ○ Stripping vapor(MSA) and heat transfer(ESA) L/V MSA (Mass-Separating Agent) V/L MSA (Mass-Separating Agent) L Ex) Separation of products from delayed coking

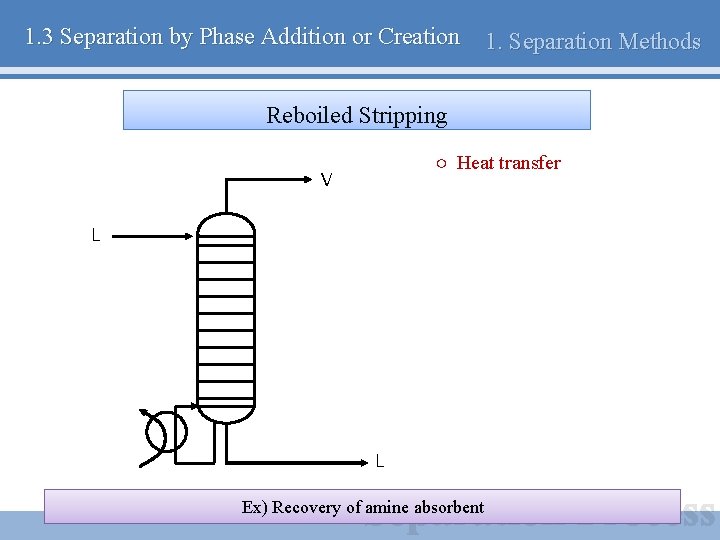
1. 3 Separation by Phase Addition or Creation 1. Separation Methods Reboiled Stripping ○ Heat transfer V L L Ex) Recovery of amine absorbent

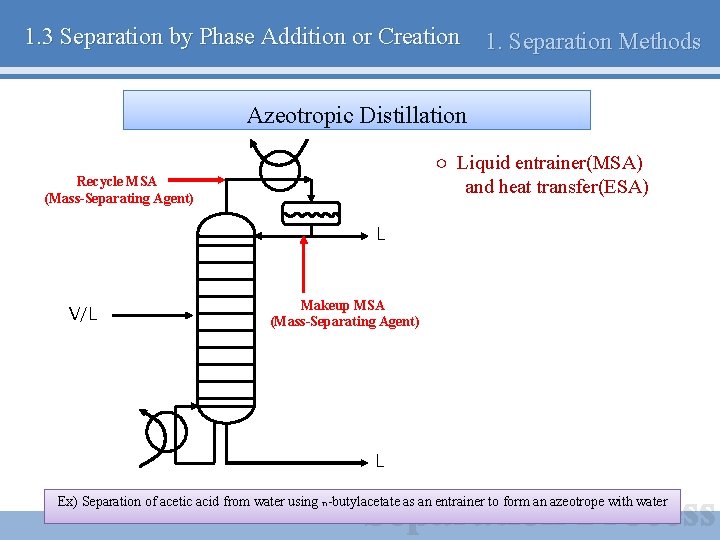
1. 3 Separation by Phase Addition or Creation 1. Separation Methods Azeotropic Distillation ○ Liquid entrainer(MSA) and heat transfer(ESA) Recycle MSA (Mass-Separating Agent) L V/L Makeup MSA (Mass-Separating Agent) L Ex) Separation of acetic acid from water using n-butylacetate as an entrainer to form an azeotrope with water

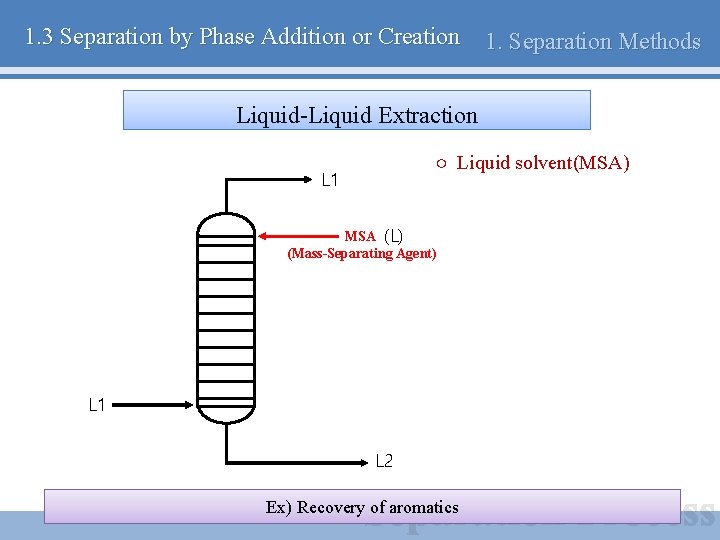
1. 3 Separation by Phase Addition or Creation 1. Separation Methods Liquid-Liquid Extraction ○ Liquid solvent(MSA) L 1 MSA (L) (Mass-Separating Agent) L 1 L 2 Ex) Recovery of aromatics

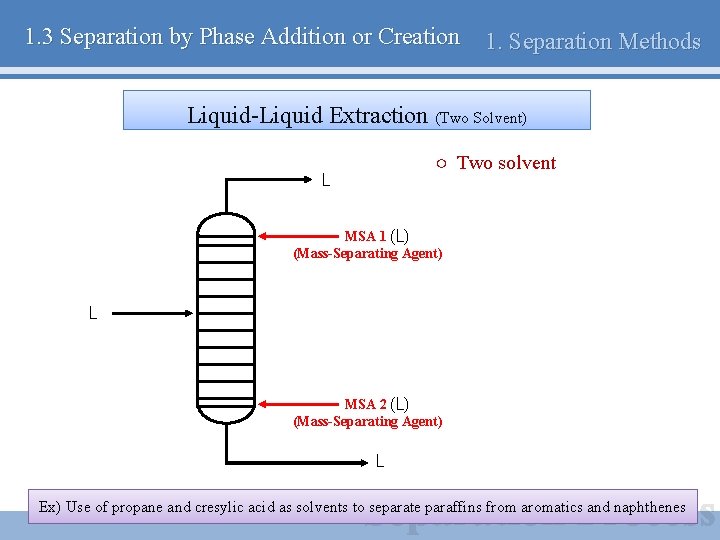
1. 3 Separation by Phase Addition or Creation 1. Separation Methods Liquid-Liquid Extraction (Two Solvent) ○ Two solvent L MSA 1 (L) (Mass-Separating Agent) L MSA 2 (L) (Mass-Separating Agent) L Ex) Use of propane and cresylic acid as solvents to separate paraffins from aromatics and naphthenes

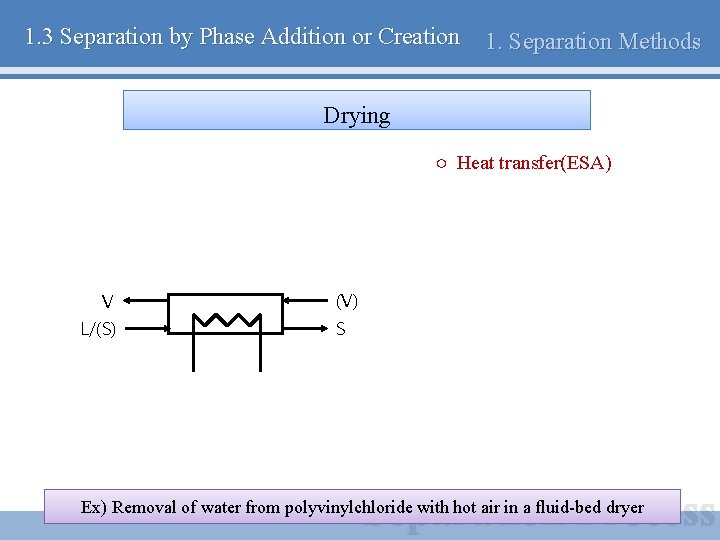
1. 3 Separation by Phase Addition or Creation 1. Separation Methods Drying ○ Heat transfer(ESA) V L/(S) (V) S Ex) Removal of water from polyvinylchloride with hot air in a fluid-bed dryer

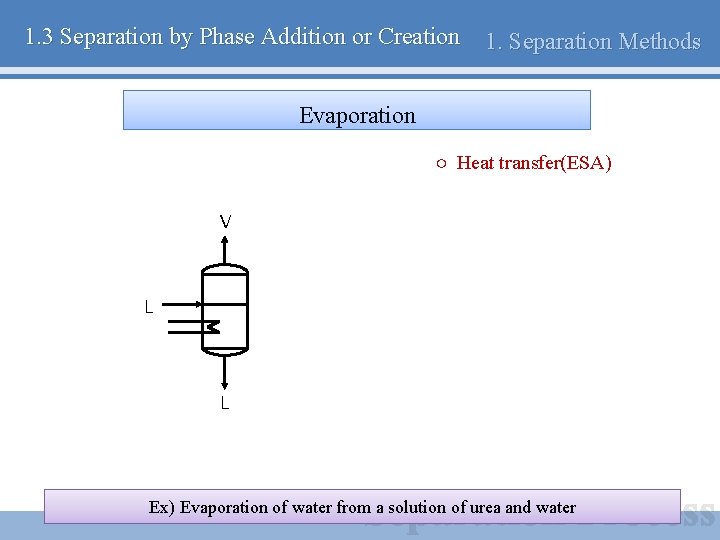
1. 3 Separation by Phase Addition or Creation 1. Separation Methods Evaporation ○ Heat transfer(ESA) V L L Ex) Evaporation of water from a solution of urea and water

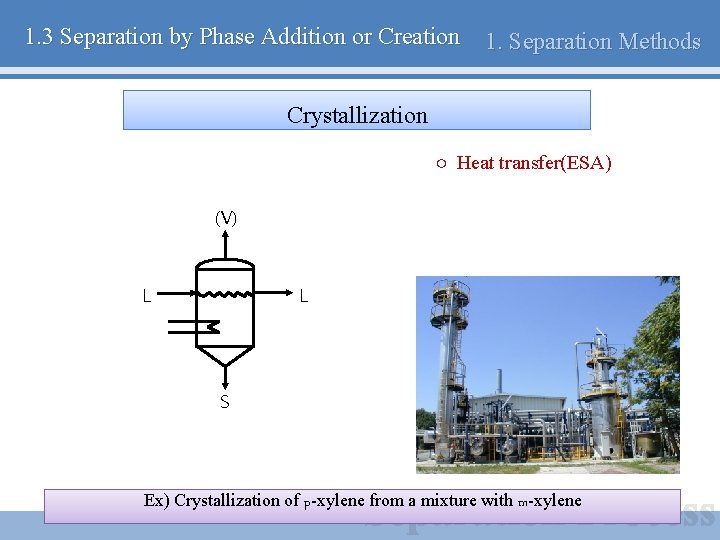
1. 3 Separation by Phase Addition or Creation 1. Separation Methods Crystallization ○ Heat transfer(ESA) (V) L L S Ex) Crystallization of p-xylene from a mixture with m-xylene

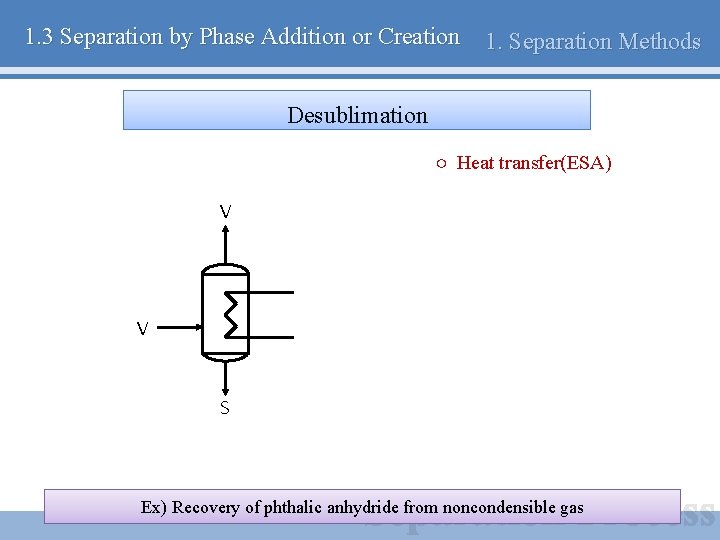
1. 3 Separation by Phase Addition or Creation 1. Separation Methods Desublimation ○ Heat transfer(ESA) V V S Ex) Recovery of phthalic anhydride from noncondensible gas

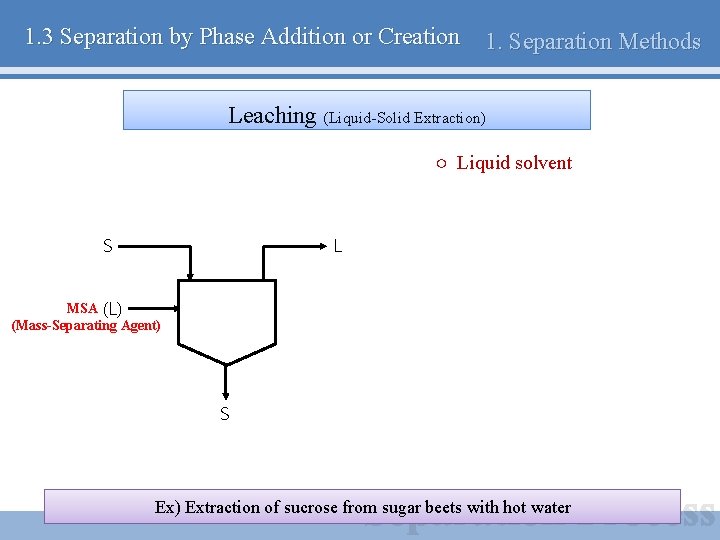
1. 3 Separation by Phase Addition or Creation 1. Separation Methods Leaching (Liquid-Solid Extraction) ○ Liquid solvent S L MSA (L) (Mass-Separating Agent) S Ex) Extraction of sucrose from sugar beets with hot water

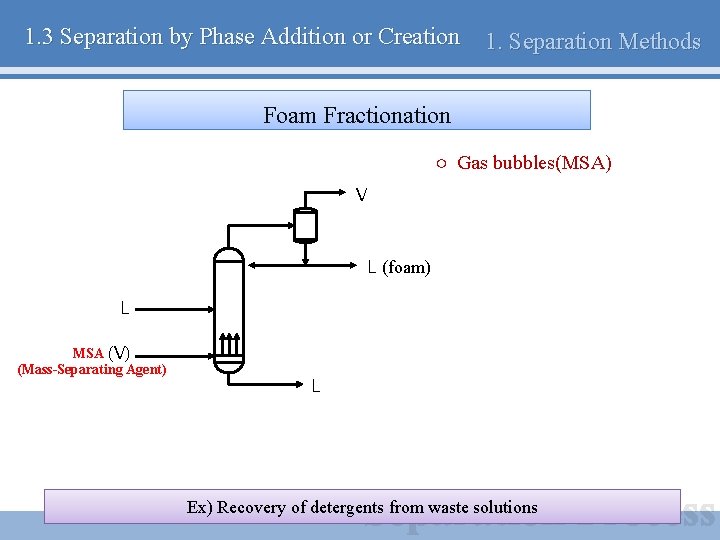
1. 3 Separation by Phase Addition or Creation 1. Separation Methods Foam Fractionation ○ Gas bubbles(MSA) V L (foam) L MSA (V) (Mass-Separating Agent) L Ex) Recovery of detergents from waste solutions

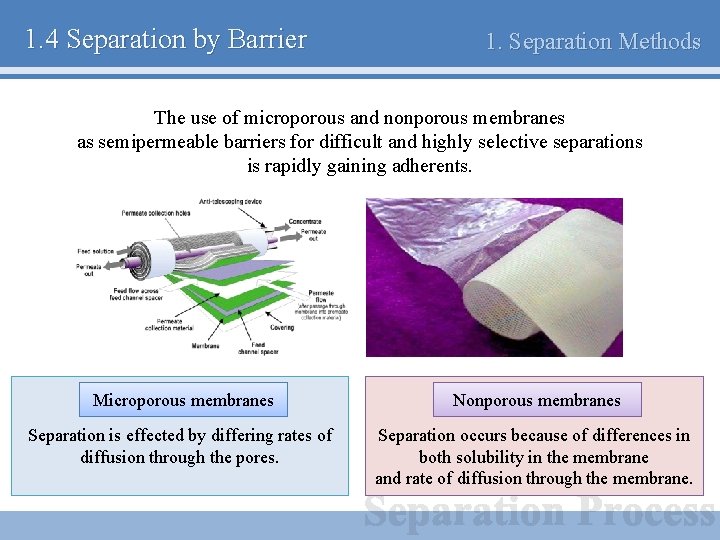
1. 4 Separation by Barrier 1. Separation Methods The use of microporous and nonporous membranes as semipermeable barriers for difficult and highly selective separations is rapidly gaining adherents. Microporous membranes Nonporous membranes Separation is effected by differing rates of diffusion through the pores. Separation occurs because of differences in both solubility in the membrane and rate of diffusion through the membrane.

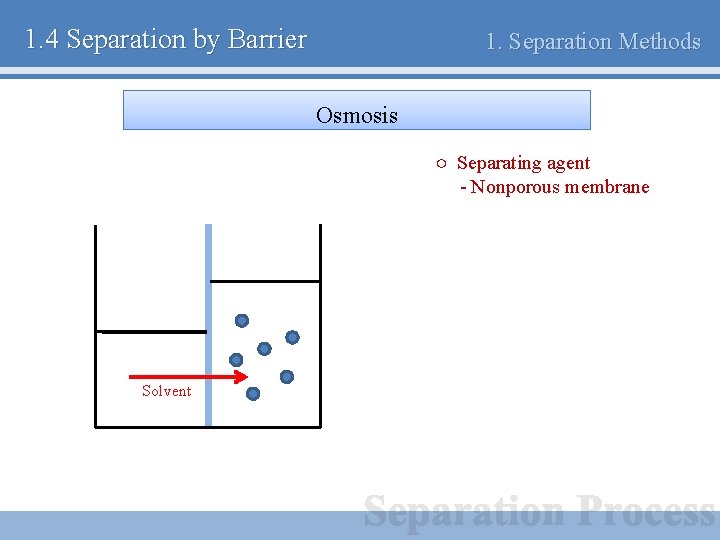
1. 4 Separation by Barrier 1. Separation Methods Osmosis ○ Separating agent - Nonporous membrane Solvent

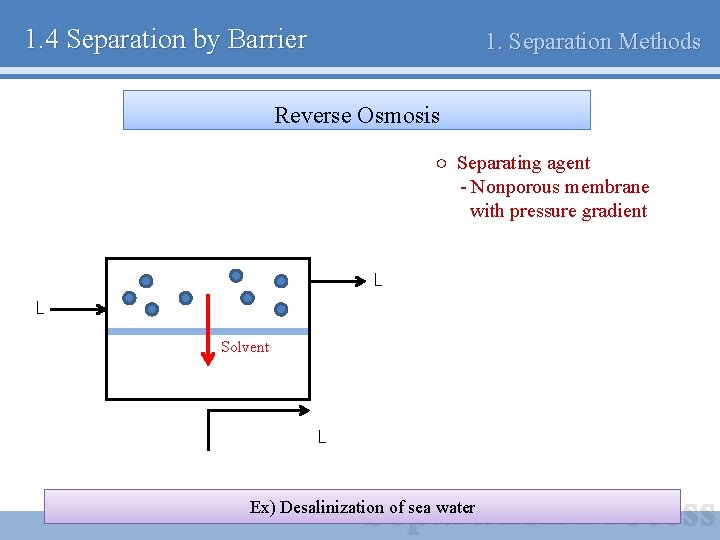
1. 4 Separation by Barrier 1. Separation Methods Reverse Osmosis ○ Separating agent - Nonporous membrane with pressure gradient L L Solvent L Ex) Desalinization of sea water

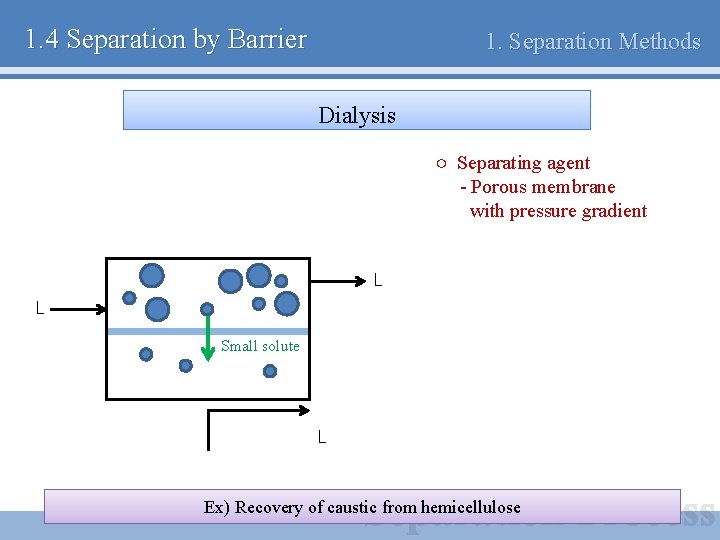
1. 4 Separation by Barrier 1. Separation Methods Dialysis ○ Separating agent - Porous membrane with pressure gradient L L Small solute L Ex) Recovery of caustic from hemicellulose

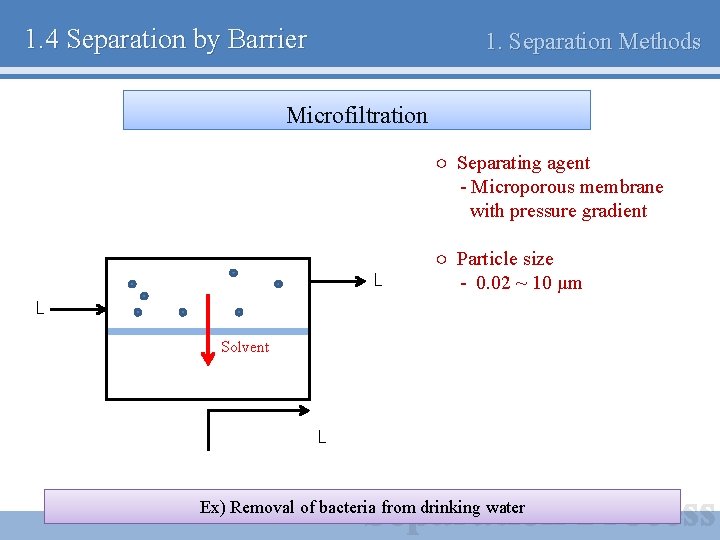
1. 4 Separation by Barrier 1. Separation Methods Microfiltration ○ Separating agent - Microporous membrane with pressure gradient L ○ Particle size - 0. 02 ~ 10 μm L Solvent L Ex) Removal of bacteria from drinking water

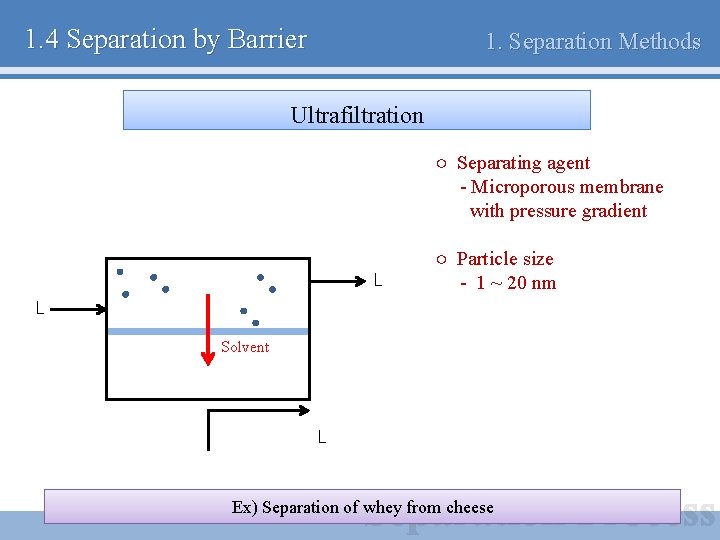
1. 4 Separation by Barrier 1. Separation Methods Ultrafiltration ○ Separating agent - Microporous membrane with pressure gradient L ○ Particle size - 1 ~ 20 nm L Solvent L Ex) Separation of whey from cheese

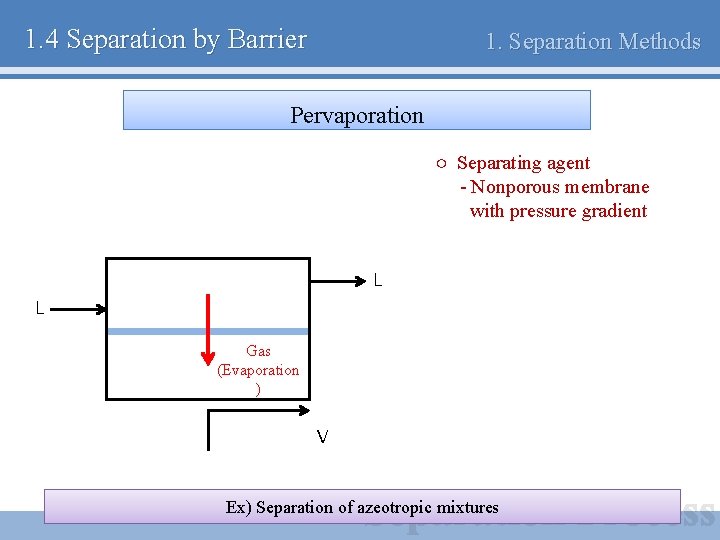
1. 4 Separation by Barrier 1. Separation Methods Pervaporation ○ Separating agent - Nonporous membrane with pressure gradient L L Gas (Evaporation ) V Ex) Separation of azeotropic mixtures

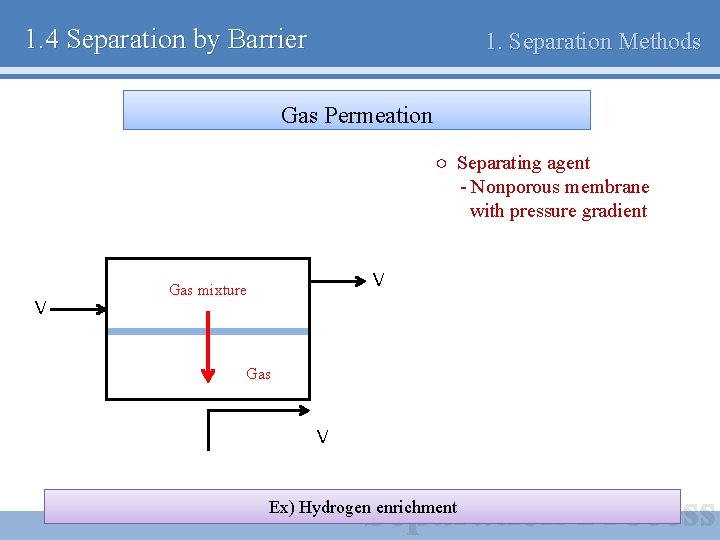
1. 4 Separation by Barrier 1. Separation Methods Gas Permeation ○ Separating agent - Nonporous membrane with pressure gradient V V Gas mixture Gas V Ex) Hydrogen enrichment

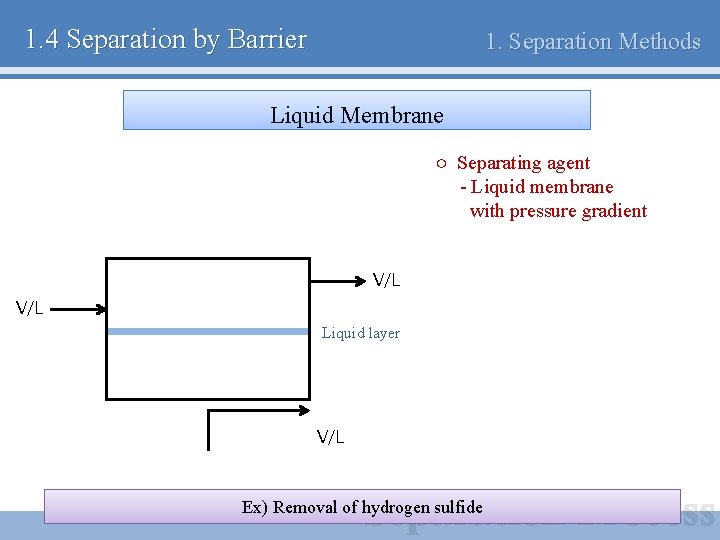
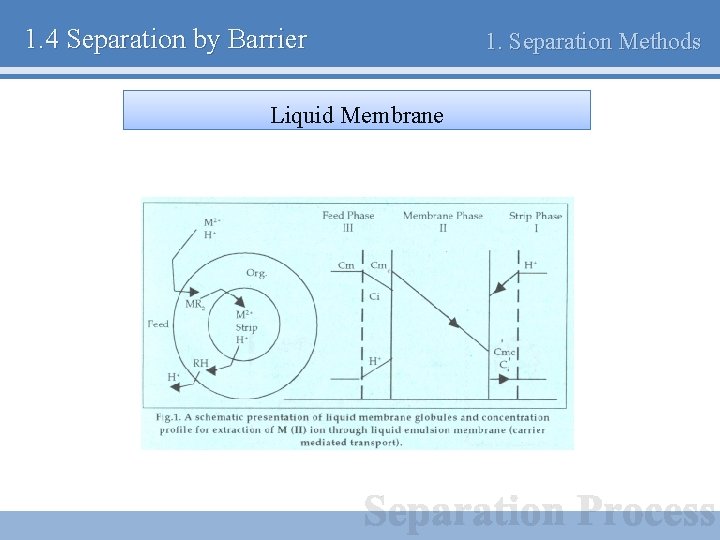
1. 4 Separation by Barrier 1. Separation Methods Liquid Membrane ○ Separating agent - Liquid membrane with pressure gradient V/L Liquid layer V/L Ex) Removal of hydrogen sulfide

1. 4 Separation by Barrier Liquid Membrane 1. Separation Methods

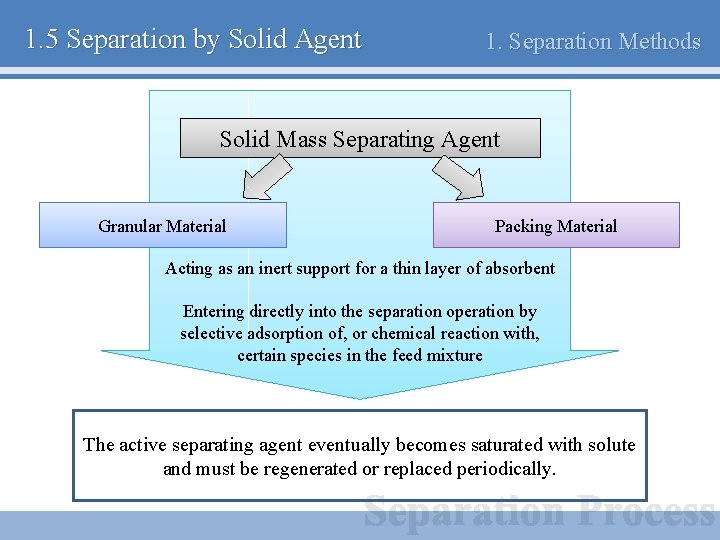
1. 5 Separation by Solid Agent 1. Separation Methods Solid Mass Separating Agent Granular Material Packing Material Acting as an inert support for a thin layer of absorbent Entering directly into the separation operation by selective adsorption of, or chemical reaction with, certain species in the feed mixture The active separating agent eventually becomes saturated with solute and must be regenerated or replaced periodically.

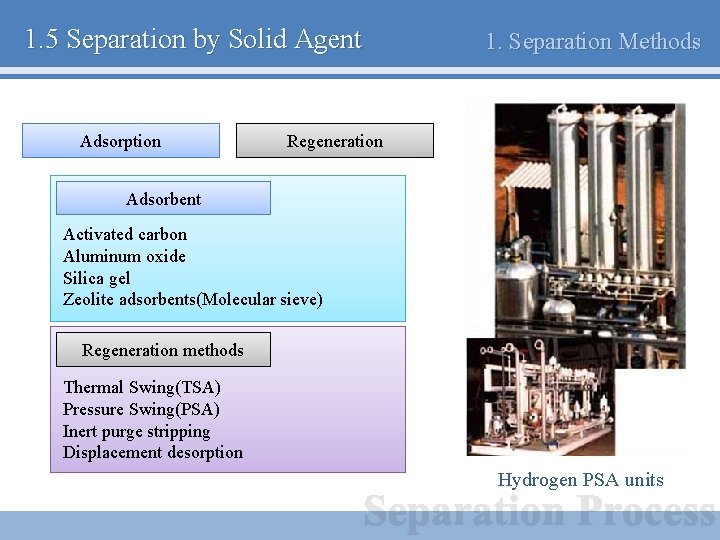
1. 5 Separation by Solid Agent Adsorption 1. Separation Methods Regeneration Adsorbent Activated carbon Aluminum oxide Silica gel Zeolite adsorbents(Molecular sieve) Regeneration methods Thermal Swing(TSA) Pressure Swing(PSA) Inert purge stripping Displacement desorption Hydrogen PSA units

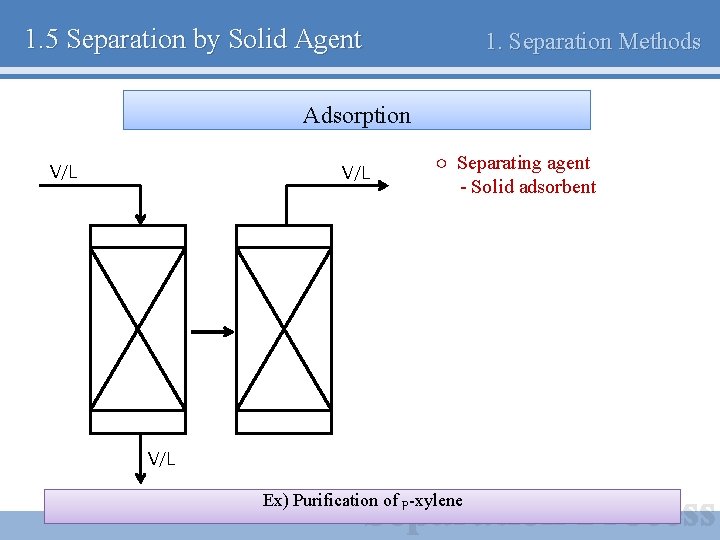
1. 5 Separation by Solid Agent 1. Separation Methods Adsorption V/L ○ Separating agent - Solid adsorbent V/L Ex) Purification of p-xylene

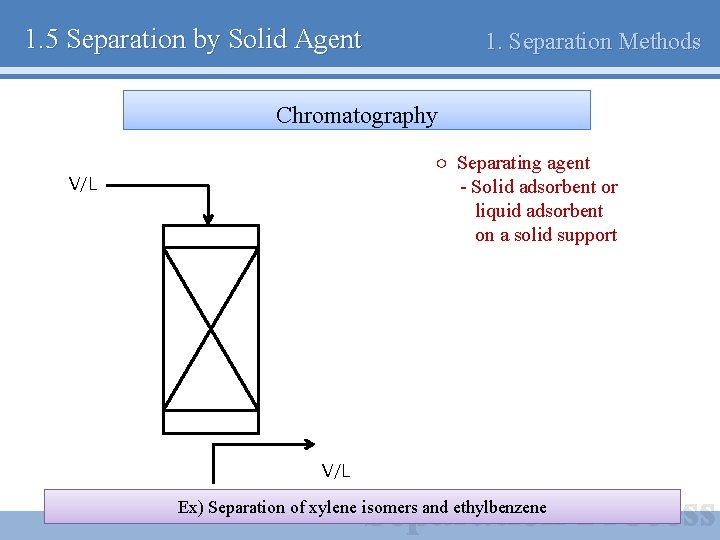
1. 5 Separation by Solid Agent 1. Separation Methods Chromatography ○ Separating agent - Solid adsorbent or liquid adsorbent on a solid support V/L Ex) Separation of xylene isomers and ethylbenzene

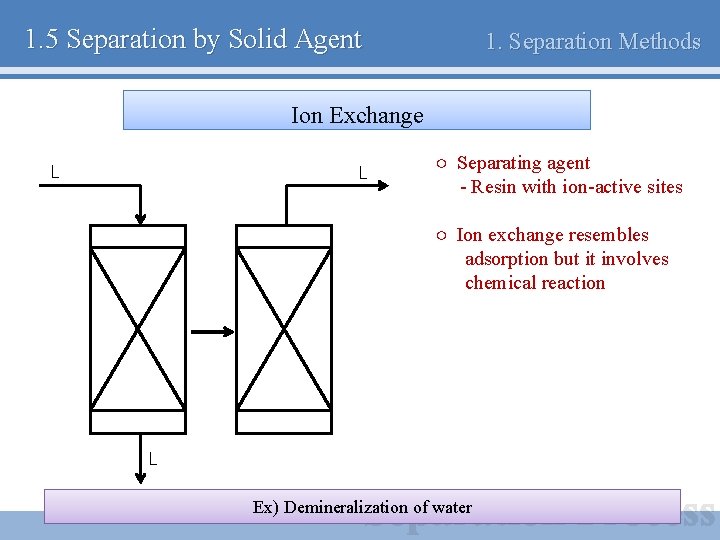
1. 5 Separation by Solid Agent 1. Separation Methods Ion Exchange L L ○ Separating agent - Resin with ion-active sites ○ Ion exchange resembles adsorption but it involves chemical reaction L Ex) Demineralization of water

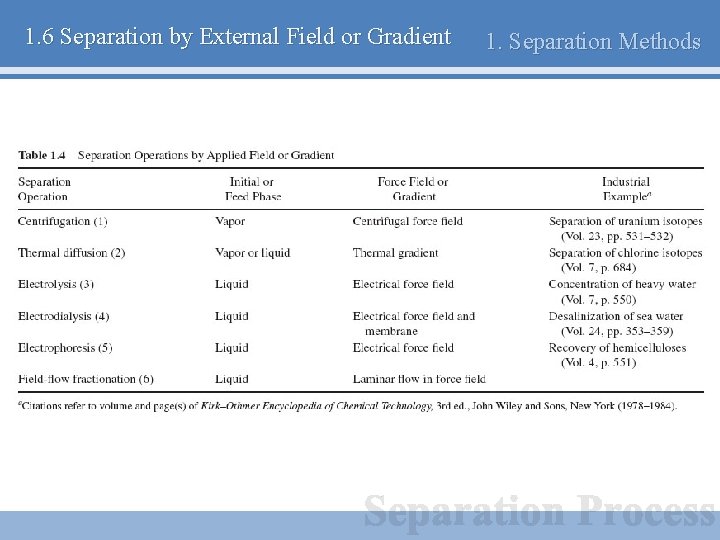
1. 6 Separation by External Field or Gradient 1. Separation Methods

1. 6 Separation by External Field or Gradient Centrifugation 1. Separation Methods

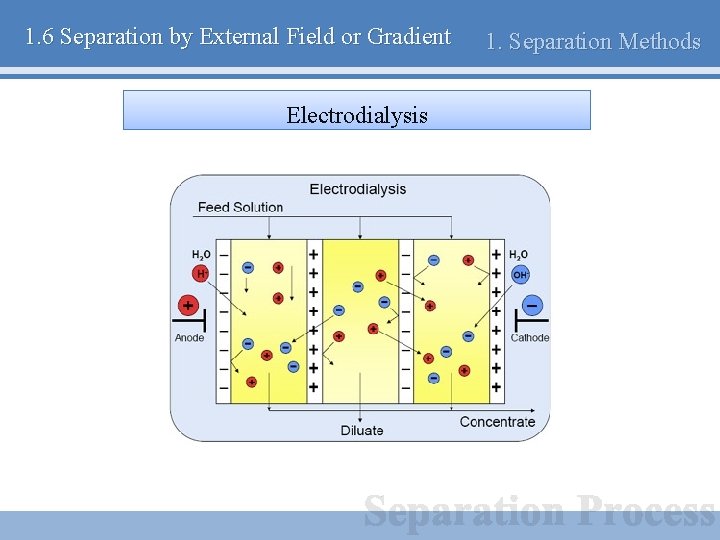
1. 6 Separation by External Field or Gradient Electrodialysis 1. Separation Methods

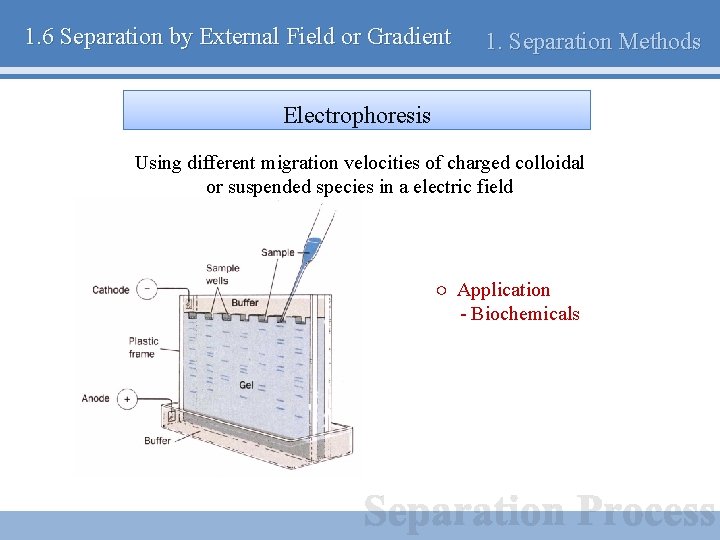
1. 6 Separation by External Field or Gradient 1. Separation Methods Electrophoresis Using different migration velocities of charged colloidal or suspended species in a electric field ○ Application - Biochemicals

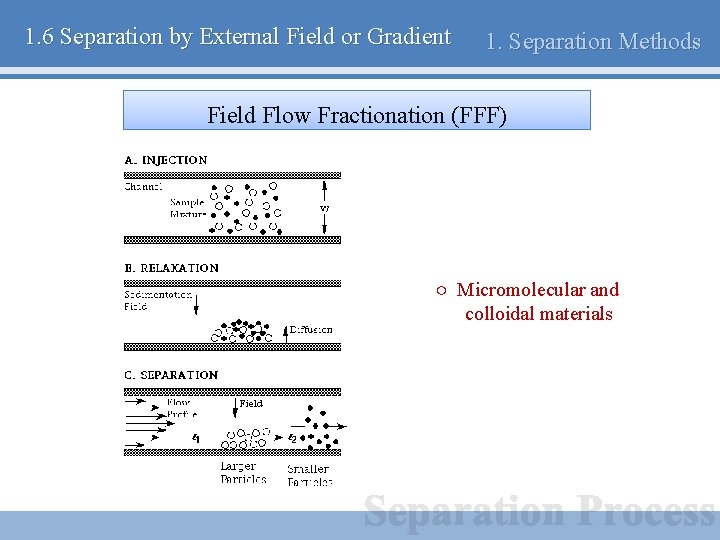
1. 6 Separation by External Field or Gradient 1. Separation Methods Field Flow Fractionation (FFF) ○ Micromolecular and colloidal materials

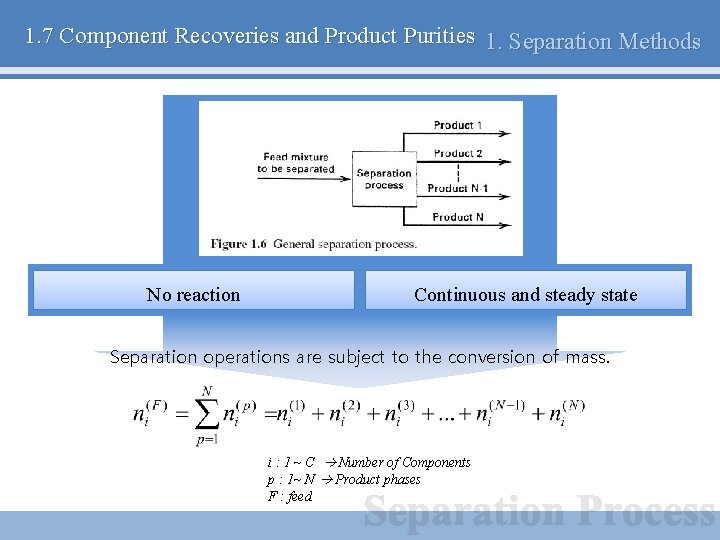
1. 7 Component Recoveries and Product Purities 1. Separation Methods No reaction Continuous and steady state Separation operations are subject to the conversion of mass. i : 1 ~ C Number of Components p : 1~ N Product phases F : feed

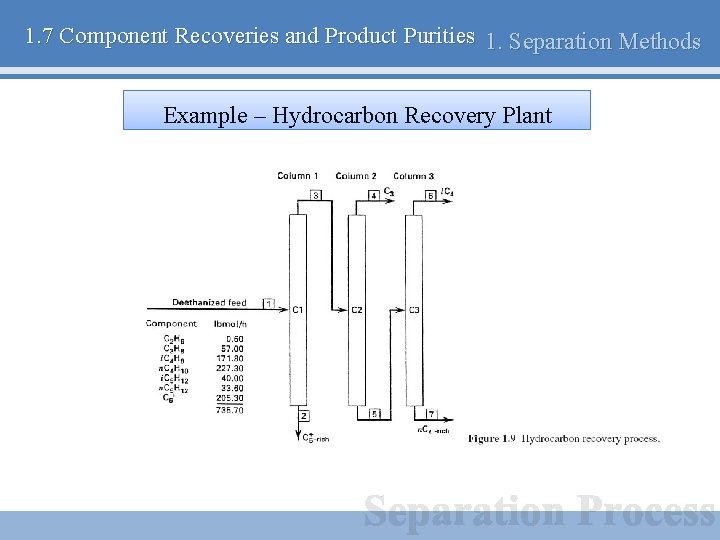
1. 7 Component Recoveries and Product Purities 1. Separation Methods Example – Hydrocarbon Recovery Plant

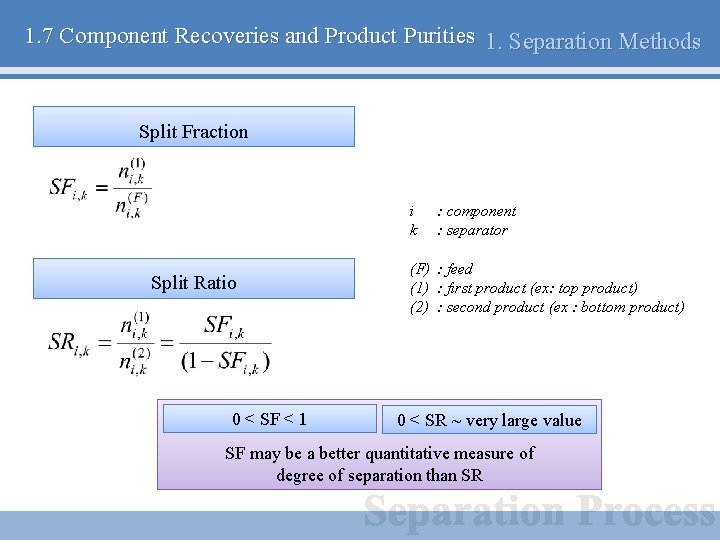
1. 7 Component Recoveries and Product Purities 1. Separation Methods Split Fraction i k Split Ratio 0 < SF < 1 : component : separator (F) : feed (1) : first product (ex: top product) (2) : second product (ex : bottom product) 0 < SR ~ very large value SF may be a better quantitative measure of degree of separation than SR

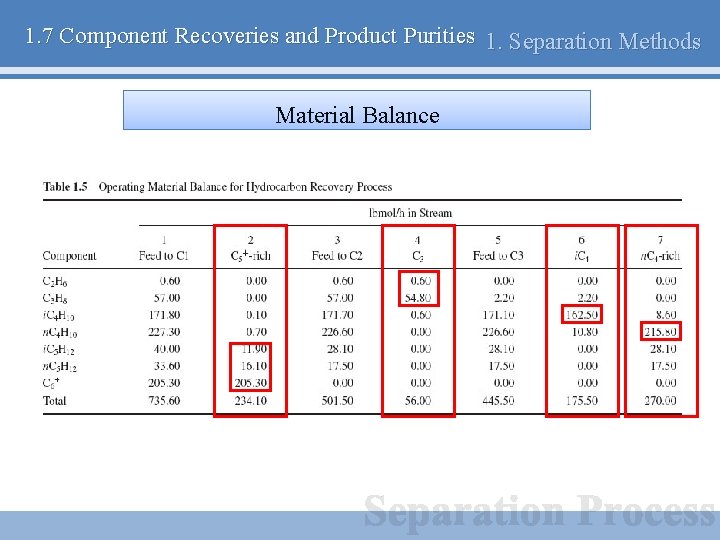
1. 7 Component Recoveries and Product Purities 1. Separation Methods Material Balance

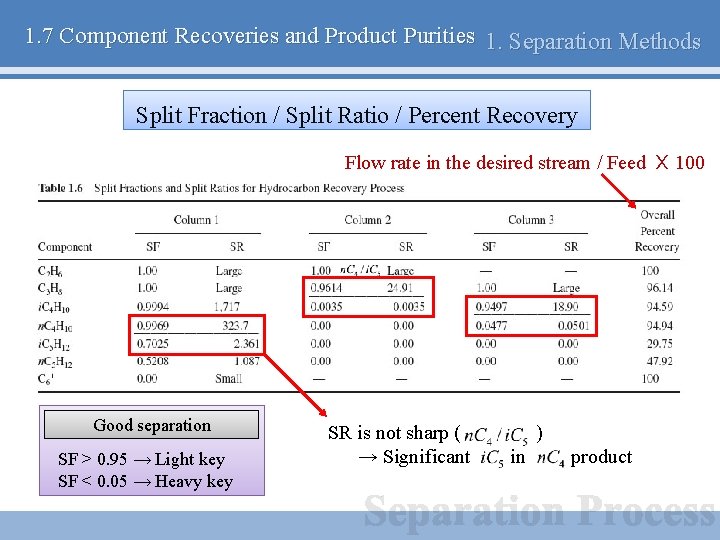
1. 7 Component Recoveries and Product Purities 1. Separation Methods Split Fraction / Split Ratio / Percent Recovery Flow rate in the desired stream / Feed X 100 Good separation SF > 0. 95 → Light key SF < 0. 05 → Heavy key SR is not sharp ( → Significant ) in product

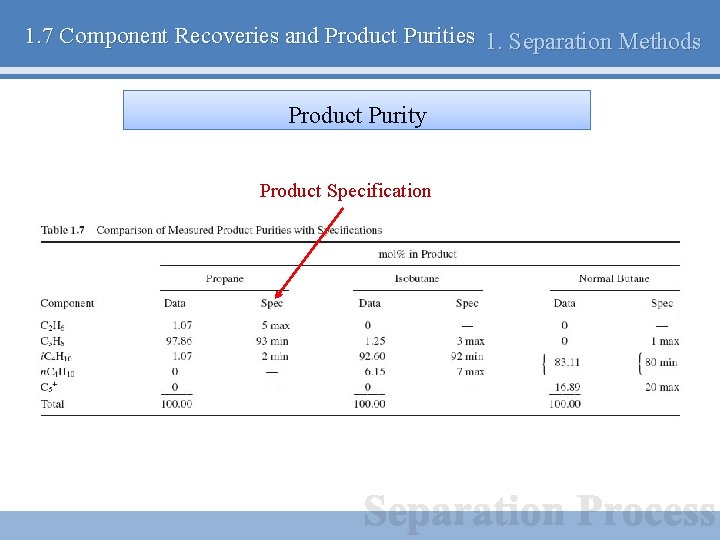
1. 7 Component Recoveries and Product Purities 1. Separation Methods Product Purity Product Specification

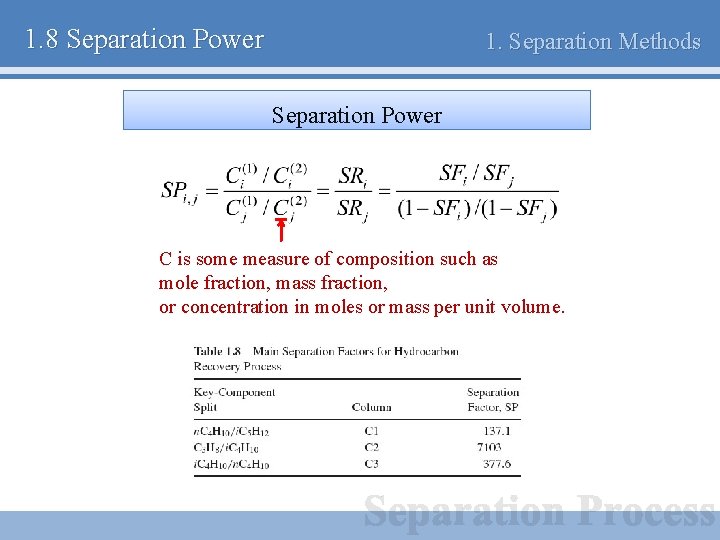
1. 8 Separation Power 1. Separation Methods Separation Power C is some measure of composition such as mole fraction, mass fraction, or concentration in moles or mass per unit volume.

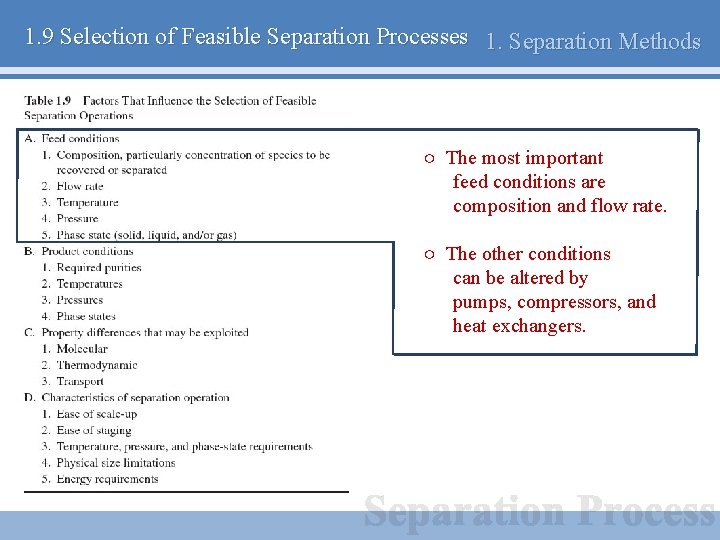
1. 9 Selection of Feasible Separation Processes 1. Separation Methods Selection of a best separation processes ○ Selection among a number of feasible candidates ○ Combination of operations may be considered (Hybrid process) Important factors that influence the selection of feasible separation operations ○ Feed conditions ○ Product conditions ○ Property differences ○ Characteristics of separation

1. 9 Selection of Feasible Separation Processes 1. Separation Methods ○ The most important feed conditions are composition and flow rate. ○ The other conditions can be altered by pumps, compressors, and heat exchangers.

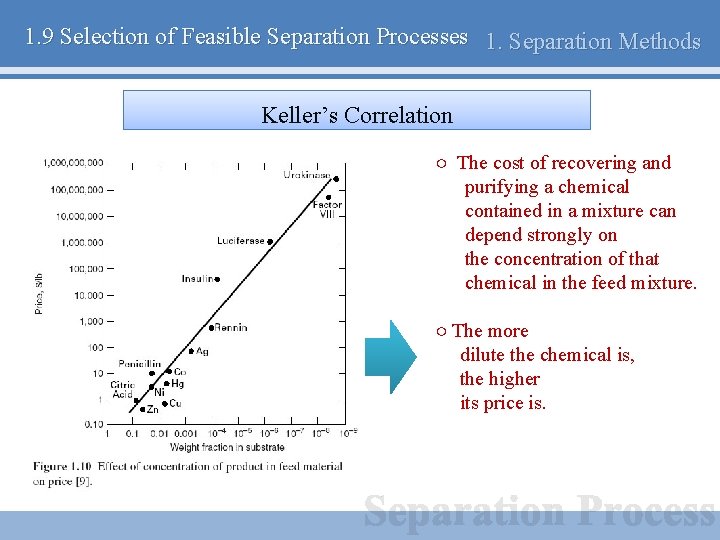
1. 9 Selection of Feasible Separation Processes 1. Separation Methods Keller’s Correlation ○ The cost of recovering and purifying a chemical contained in a mixture can depend strongly on the concentration of that chemical in the feed mixture. ○ The more dilute the chemical is, the higher its price is.

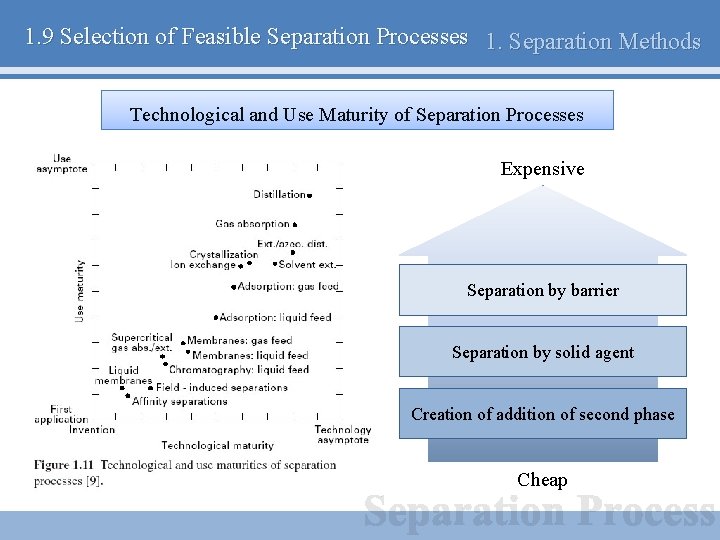
1. 9 Selection of Feasible Separation Processes 1. Separation Methods Technological and Use Maturity of Separation Processes Expensive Separation by barrier Separation by solid agent Creation of addition of second phase Cheap

1. 9 Selection of Feasible Separation Processes 1. Separation Methods Ease of Scale-up 200% 150%