Chemistry SEPARATION TECHNIQUES Separation Techniques Involve the separation








- Slides: 12

Chemistry SEPARATION TECHNIQUES

Separation Techniques Involve the separation of mixtures to enhance purity of substances Are important because most substances are needed in their pure state.

Some Types of Separation Techniques Filtration Chromatography Distillation Fractional Crystallization Centrifugation

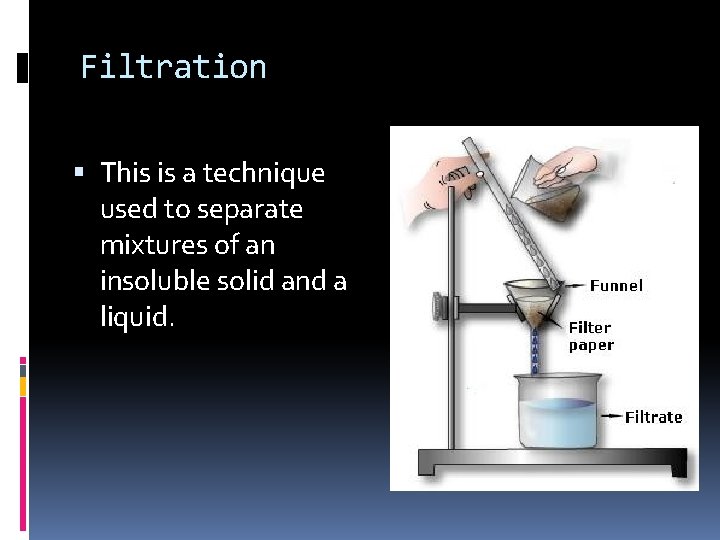
Filtration This is a technique used to separate mixtures of an insoluble solid and a liquid.

Examples of Mixtures You Can Separate Using Filtration Sand water Broken glass and a liquid A precipitate from a liquid

Chromatography Is the separation technique used to separate soluble substances using a media and a solvent. It is mostly applied in identifying mixtures that are colored or pigmented.

Distillation This is the separation technique where two miscible liquids are separated. It is made possible due to the fact that each liquid has its unique boiling point. Types of Distillation Simple distillation Fractional distillation

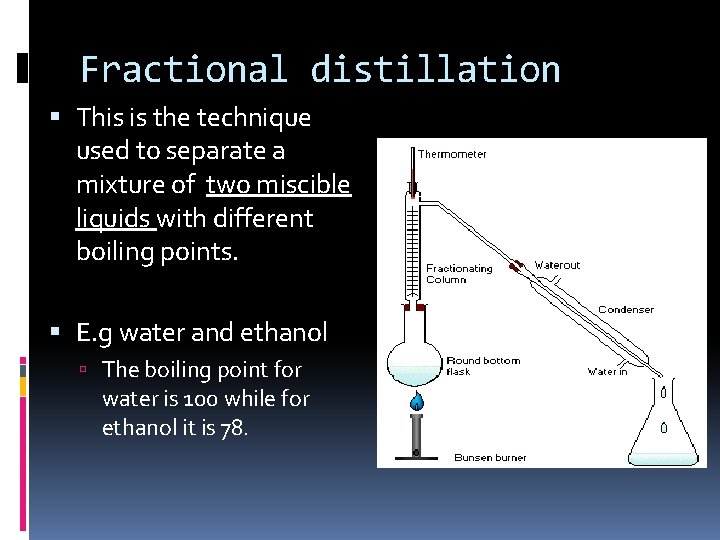
Fractional distillation This is the technique used to separate a mixture of two miscible liquids with different boiling points. E. g water and ethanol The boiling point for water is 100 while for ethanol it is 78.

Fractional Crystallization A process by which a chemical compound is separated into by crystallization. components In fractional crystallization the compound is mixed with a solvent, heated, and then gradually cooled so that, as each of its constituent components crystallizes, it can be removed in its pure form from the solution.

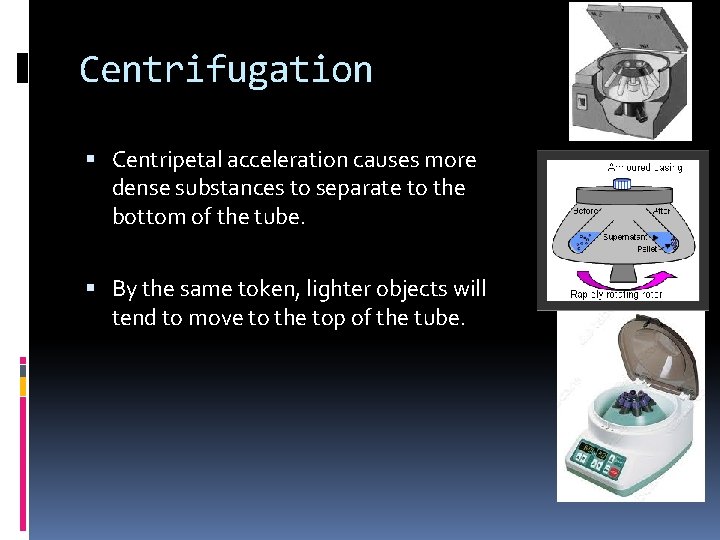
Centrifugation Centripetal acceleration causes more dense substances to separate to the bottom of the tube. By the same token, lighter objects will tend to move to the top of the tube.

Questions 1. What is a mixture? 2. Give three ways of separating a mixture. 3. What kind of mixtures can you separate using the filtration method? 4. Give an example of a mixture you can separate using the fractional distillation method.

Answers 1. It is a substance made up of two or more substances which are physically combined. 2. Filtration, Chromatography and Distillation. 3. Mixtures of an insoluble substance and a liquid. 4. Ethanol and water.