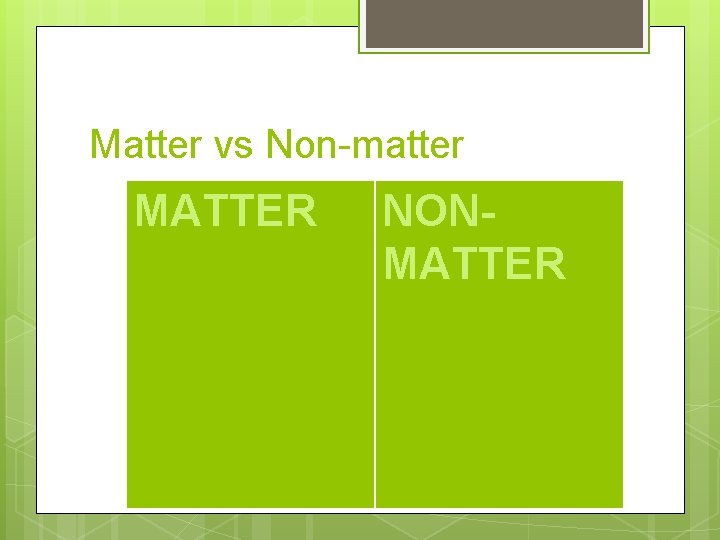
Matter Chap 3 Matter vs Nonmatter MATTER NONMATTER
























- Slides: 32

Matter- Chap 3

Matter vs Non-matter MATTER NONMATTER

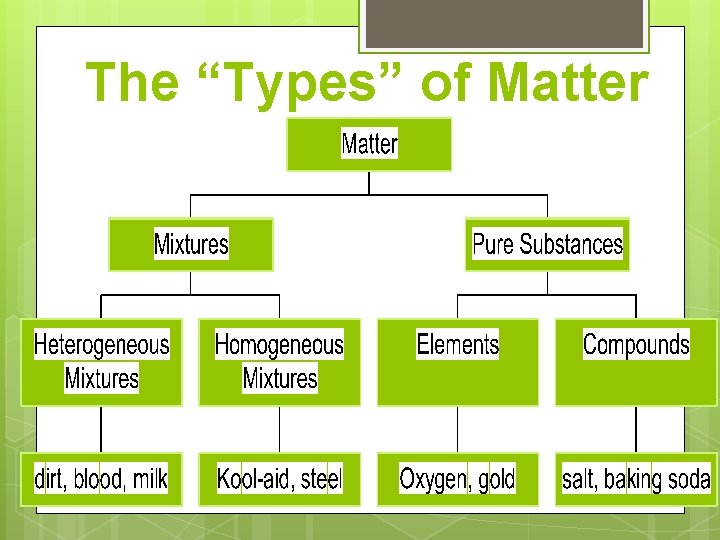
The “Types” of Matter

What is Matter? Matter - has mass and volume.

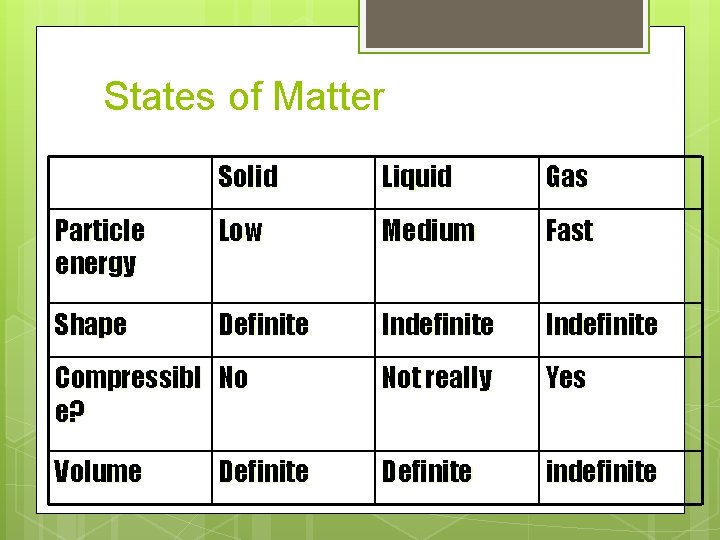
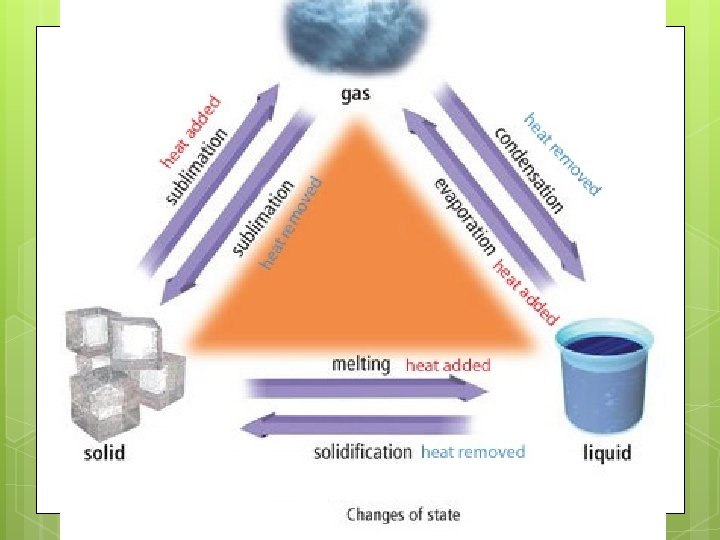
States of Matter Solid Liquid Gas Particle energy Low Medium Fast Shape Definite Indefinite Compressibl No e? Not really Yes Volume Definite indefinite Definite

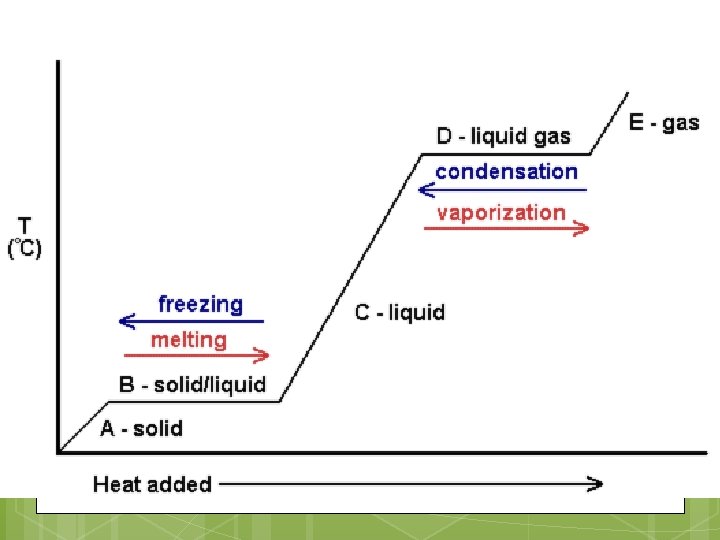
Phase Diagram


Not all matter has the same melting/boiling temperatures! http: //www. kentchemistry. com/links/Matter/Ph ase. Changes. htm

Matter can be sub-classed as pure substances and mixtures. A fish tank with fish, water, plants, shells, and gravel is a mixture. Water is a pure substance.

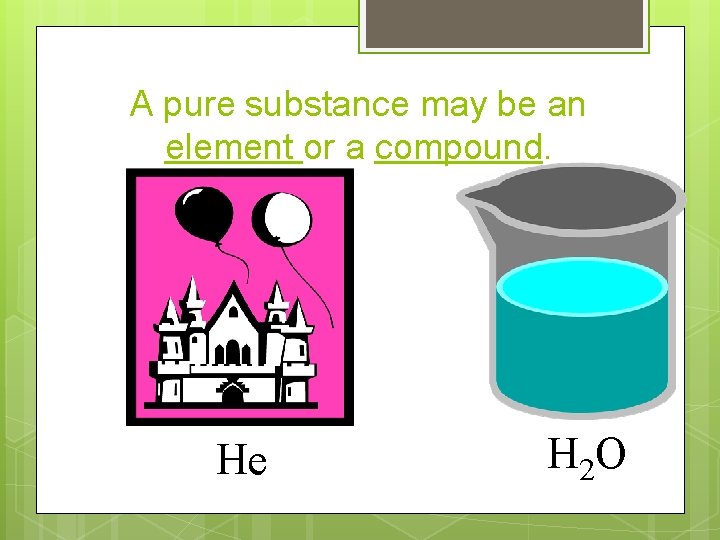
Pure substance A pure substance is composed of only one kind of atom or molecule. An atom is the basic unit of matter. A molecule is a group of atoms held together by chemical bonds.

A pure substance may be an element or a compound. He H 2 O

Elements are made up by only one type of atom Examples = He, Au, Li, F 2, H 2 , C 60

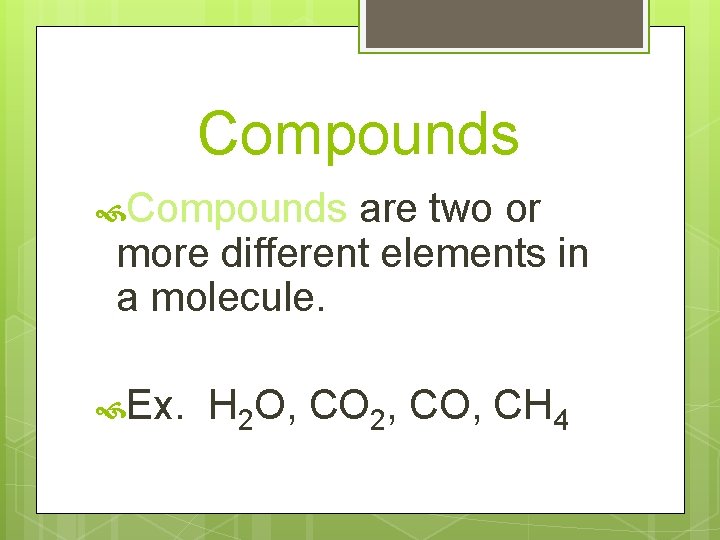
Compounds are two or more different elements in a molecule. Ex. H 2 O, CO 2, CO, CH 4

Compounds Compound properties are different from those of the elements that compose them. Ex. H 2 O = Water

Compounds can only be separated into elemental components by chemical means.

Matter can be sub-classed as pure substances and mixtures. Mixtures are not considered pure substances, but may be made up of pure substances

Mixtures The substances that make up a mixture keep their individual chemical and physical properties. Mixtures can be separated into its components by physical means.

Mixtures are subclassed as heterogeneous mixtures and homogeneous mixtures

Homogeneous Mixtures Are the same through out You can not see the individual parts of the mixtures. Example: Air, sugar water Can be sub-classed as solutions and alloys

Solutions All solutions are homogeneous mixtures. Ex. Sugar water, salt water The particles that make up a solutions are uniformly distributed.

Solutions The particles are so small that they cannot be seen even at high magnification. Examples: sweet tea, sea water, rubbing alcohol, cola

Parts of a Solution Solute = The substance being dissolved Solvent = The dissolving agent Ex. Tea with sugar = solute sugar solvent tea

Alloys A solid or liquid homogenous mixture of two or more metals. 18 karat Au is 18 parts Au out of 24. The remaining parts are Cu, Ag, or Ni.

Mixtures are subclassed as heterogeneous mixtures and homogeneous mixtures

Heterogeneous Mixtures Are not uniform through out Some heterogeneous mixtures appear homogenous to the naked eye. Ex. Skin

Heterogeneous Mixtures Examples shake

Heterogeneous Mixtures may be subclassed at suspensions and colloids.

Suspensions Have relatively large, easily seen particles that can settle out or form layers within a liquid. Ex. Medicine that says “Shake before Using”

Suspensions Particles can be filtered out in water suspensions

Colloids A colloid has tiny particles, just large enough to produce a cloudy appearance. The particles of a colloid are smaller than a suspension’s and larger than a solution’s particles.

Colloids Examples: Milk Mayonnaise

Colloids produce the Tyndall effect The Tyndall effect happens when a light beam shines through a colloid. The beam’s path can be seen in the liquid.