Separating Mixtures Separating Mixtures Most matter naturally exists

















- Slides: 20

Separating Mixtures

Separating Mixtures • Most matter naturally exists in the form of mixtures • Use can use processes based on physical properties to separate both homogenous and heterogeneous mixtures. • We will study some of the many ways to separate mixtures • Pay attention because at the end you will need to use these techniques in the lab!

Physical Separation Techniques 1. By eye – you can easily separate the M&Ms, nuts and other snacks in this mixture by eye

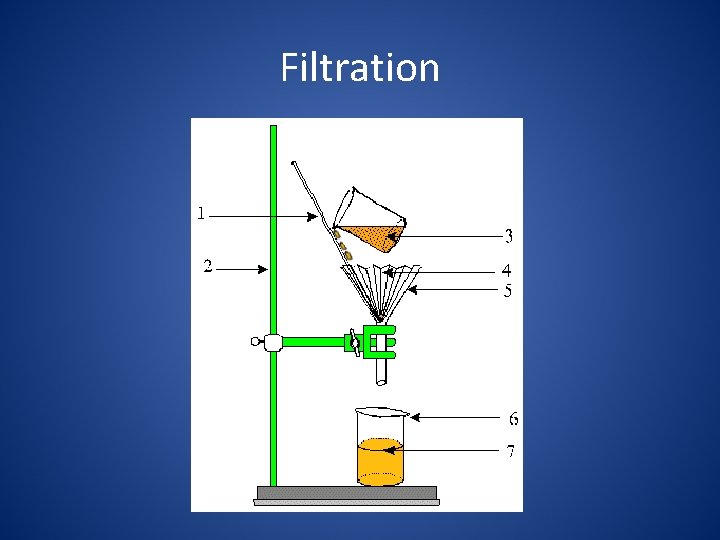
Filtration 2. Filtration uses a porous barrier to separate a solid from a liquid.

Filtration

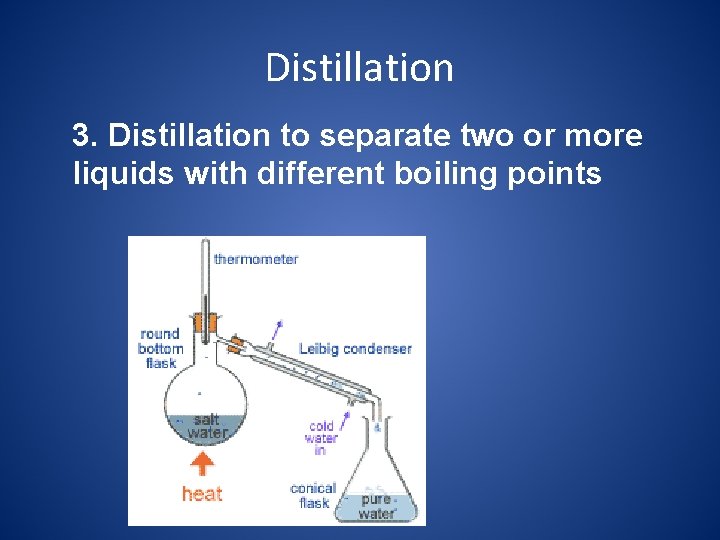
Distillation 3. Distillation to separate two or more liquids with different boiling points

Distillation Commercial still Copper alcohol still

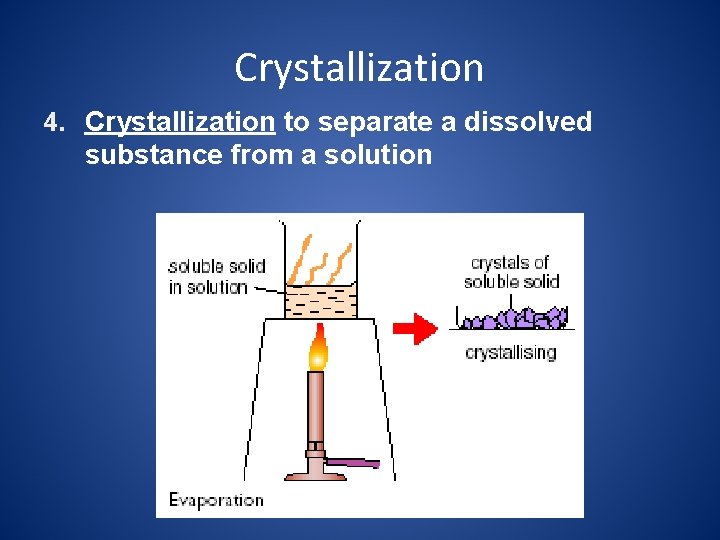
Crystallization 4. Crystallization to separate a dissolved substance from a solution

Crystallization

Magnetic separation 5. Magnetic Separation used to separate two solids when one is magnetic

Sublimation 6. Sublimation is used to separate two solids when one sublimates and the other does not.

Chromatography 7. Chromatography separates based on the ability of each component to travel or be drawn across the surface of another material.

Centrifuging 8. Often used to separate blood, the centrifuge uses the principle that some parts of mixtures are heavier than others.

Check for Understanding • What separation technique would you used to separate: – Two colorless liquids – A nondissolving solid mixed with a liquid – Red and blue marbles of the same size and mass

Now its your turn! • Separating out the different components in a mixture can often prove quite challenging, yet separation and recovery are extremely important operations both for research and for industry.

Now its your turn • Use the information about separation techniques you just learned about and design a procedure to separate a mixture of: sand, salt, poppy seeds, styrofoam balls, and iron filings.

Now its your turn • Obtain a sample of the mixture and examine it carefully. • Place a small, representative portion of the mixture (no more than 20% of what you are given) in the top left corner of a microwell plate.

Now its your turn! • Using any materials you want (within reason), develop and implement a procedure that enables you to separate out the mixture and recover all five components, each in as pure and dry a state as possible. • Helpful Hint: If water is part of your procedure, be careful not to use too much!

Now its your turn! • As each component is separated off, place it in a small plastic sack or microplate well. • Be sure to keep careful notes as you will be writing up the purpose, materials list, procedure, and conclusions for this lab.

Concluding Questions • What made you decide to do your procedure steps in the order that you did them? Would any order have worked? • If you were to do the lab over again, what specifically might you do differently? • For each of the components, describe a specific physical property that enabled you to separate it from the rest of the mixture.