Elements Compounds Mixtures Matter Mixtures Multiple substances Pure

Elements, Compounds & Mixtures
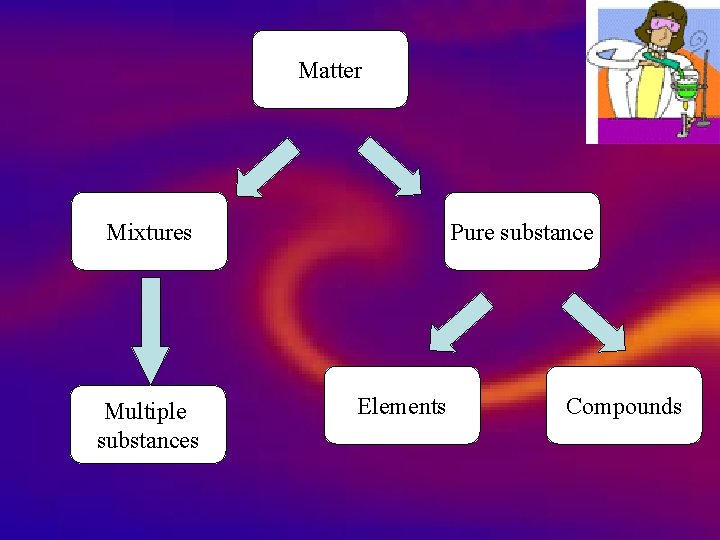
Matter Mixtures Multiple substances Pure substance Elements Compounds
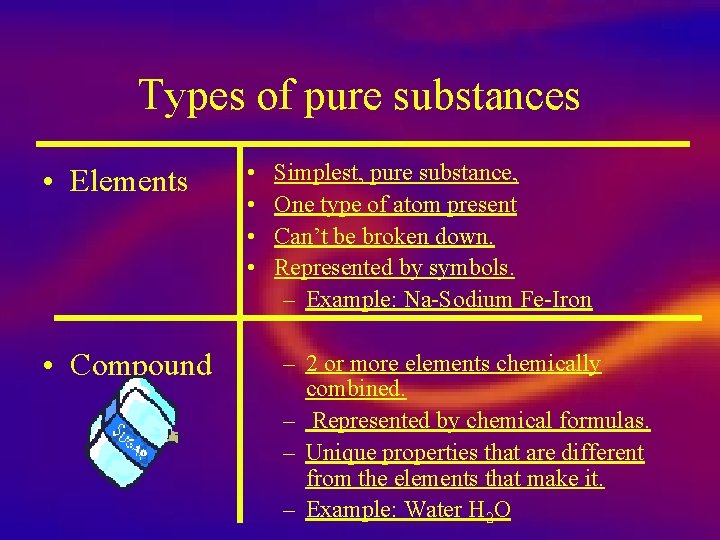
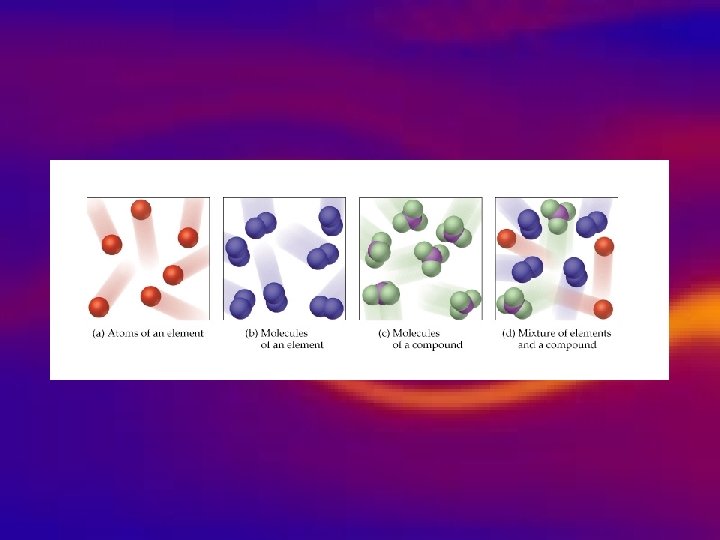
Types of pure substances • Elements • Compound • • Simplest, pure substance, One type of atom present Can’t be broken down. Represented by symbols. – Example: Na-Sodium Fe-Iron – 2 or more elements chemically combined. – Represented by chemical formulas. – Unique properties that are different from the elements that make it. – Example: Water H 2 O

Types of Mixtures • Mixtures • 1. Made of more than one kind of pure substance. • 2. Can be separated or pulled apart physically ( they aren’t chemically combined) • 3. 2 types: – Heterogeneous – Homogeneous

Describe in a T-Chart • Pure substances • Mixtures

Heterogeneous Mixtures • 1. Not the same throughout. • 2. Large easily seen particles that can be separated out. • 3. Heterogeneous liquid mixtures are also known as suspensions. – Settles after standing. • Examples: salad, Italian dressing,

Homogeneous Mixtures • Homogeneous • 1. Appears same throughout • 2. Well mixed Mixtures • 3. Will not completely settle upon standing, • 4. Tiny unrecognizable particles • 5. Homogenous mixtures are also called Solutions. • Examples: Kool-aid
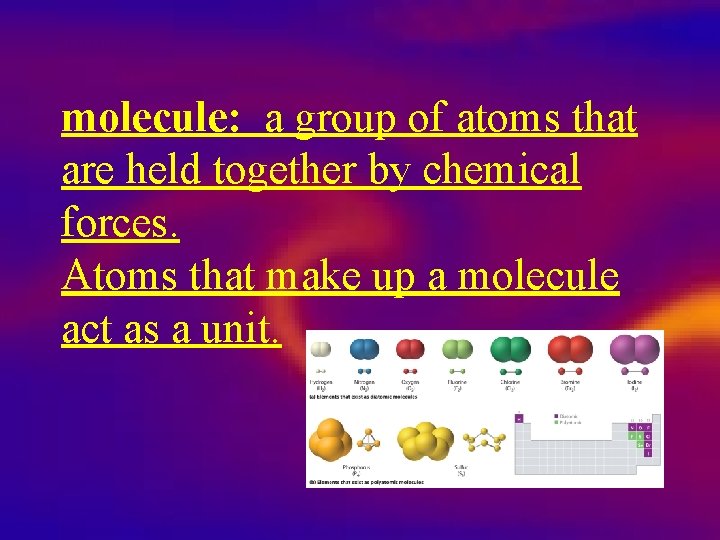
molecule: a group of atoms that are held together by chemical forces. Atoms that make up a molecule act as a unit.
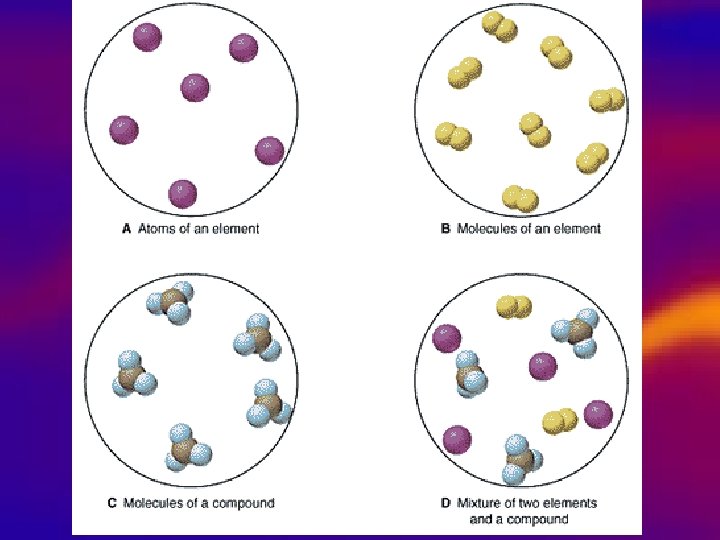
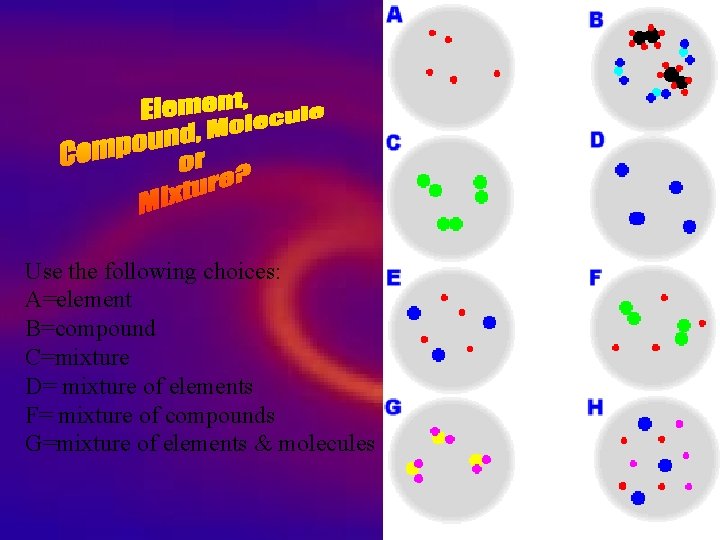
Use the following choices: A=element B=compound C=mixture D= mixture of elements F= mixture of compounds G=mixture of elements & molecules

Make a T-Chart to compare and contrast the two types of mixtures • Homogeneous Mixture • Heterogeneous mixture
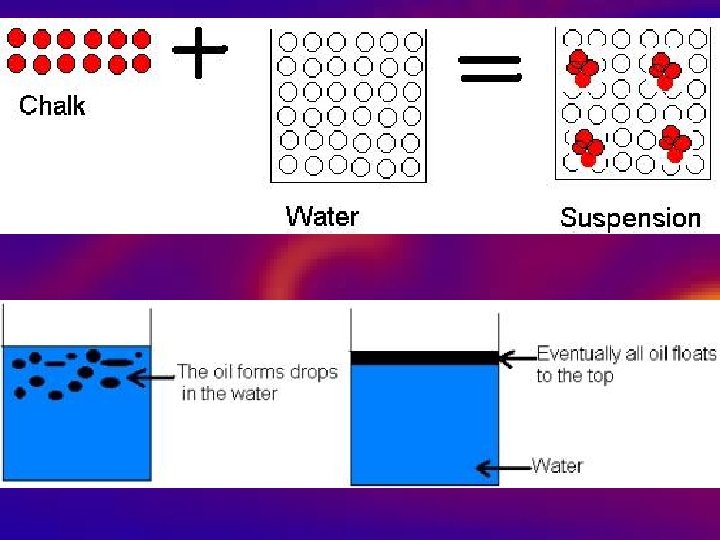


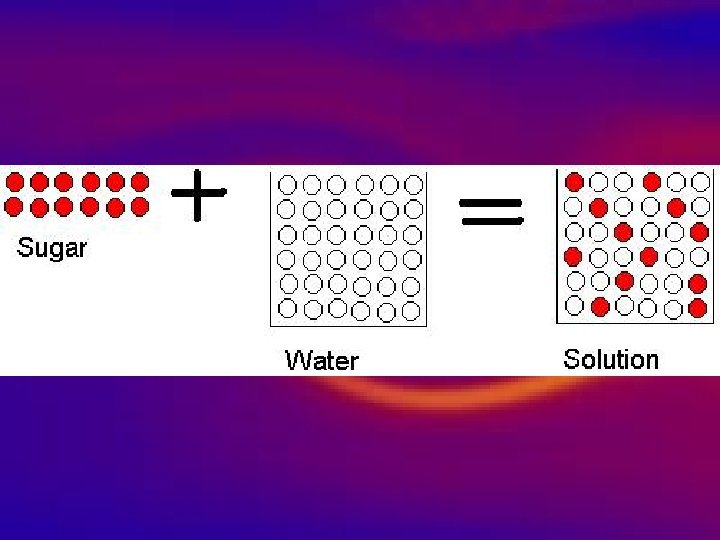

Mixture Question? • What is the difference between a heterogeneous and homogeneous mixture?
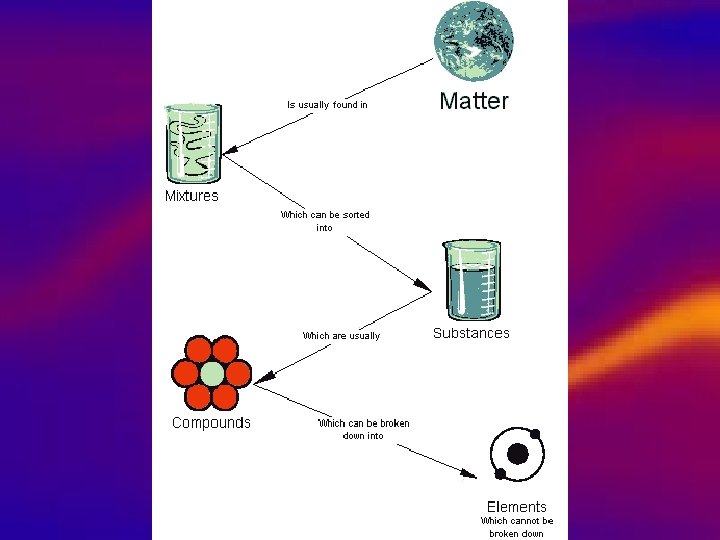


Graphic Organizer • Create a graphic organizer to show the relationship between the following terms: – – – – elements compounds homogeneous mixtures heterogeneous mixtures suspensions colloids solutions
- Slides: 20