SOLUTIONS A homogeneous mixture in which the components




















- Slides: 38

SOLUTIONS A homogeneous mixture in which the components are uniformly intermingled

Colligative property Concentration Dilute solution Vocabulary Electrolyte Terms to Know Immiscible Molarity Saturated solution vs Unsaturated vs Supersaturated Solubility Solute Solvent

ELECTROLYTES l l l Substances that break up in water to produce ions. These ions can conduct electric current Examples: Acids, Bases and Salts (ionic compounds)

ELECTROLYTES l l When substances break up into their ions in water, this process is called ionization or dissociation. For these compounds, we can write their dissociation equation: Ba. Cl 2(s) Ba 2+(aq) + 2 Cl– (aq) Pb(NO 3)2(s) Pb 2+(aq) + 2 NO 3–(aq)

Terms Solvent – The substance present in the largest amount in a solution. The substance that does the dissolving. Solute – The other substance or substances in a solution. The substance that is dissolved.

SOLUBILITY l Is the amount of a substance that dissolves in a given quantity of solvent (usually water) at a specific temperature to produce a saturated solution

SOLUBILITY of Polar vs Non. Polar l “Like dissolves Like” – Polar molecules dissolve polar molecules (Ionic compounds dissolve ionic compounds) – Nonpolar molecules dissolve nonpolar molecules (Molecular compounds dissolve molecular compounds) Lab/Demo for Electrolytes

SOLUBILITY RULES l l l All common salts of Group I elements and ammonium are soluble All common acetates and nitrates are soluble All binary compounds of Group 7 (other than F) with metals are soluble except those of silver, mercury I and lead All sulfates are soluble except those of barium, strontium, calcium, silver, mercury I and lead Except for those in Rule 1, carbonates, hydroxides, sulfides and phosphates are insoluble

Super Cooled Water Terms l Saturated – l When a solution contains the maximum amount of solute Unsaturated l – Concentrated l – When a relatively large amount of solute is dissolved Dilute l l When a solvent can dissolve more solute When a relatively small amount of solute is dissolved Supersaturated – When the solution contains more solute than a saturated solution will hold at that temperature

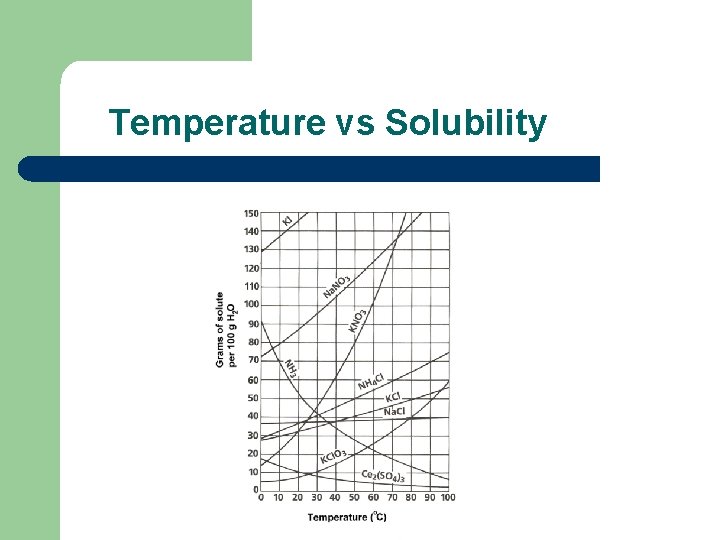
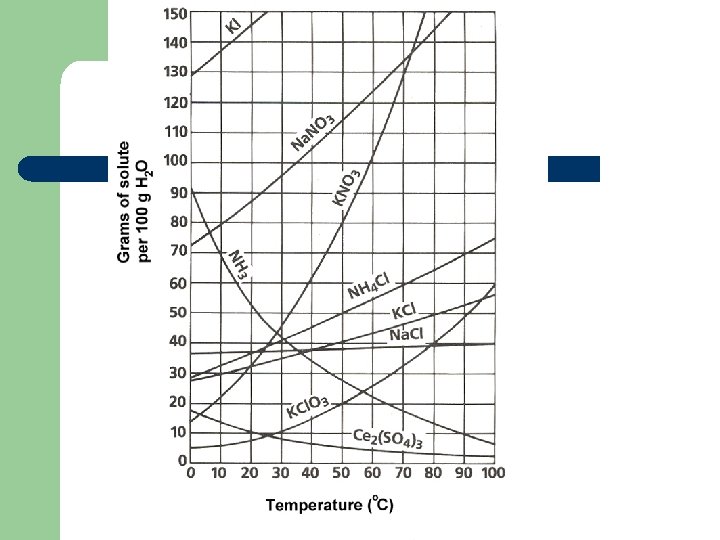
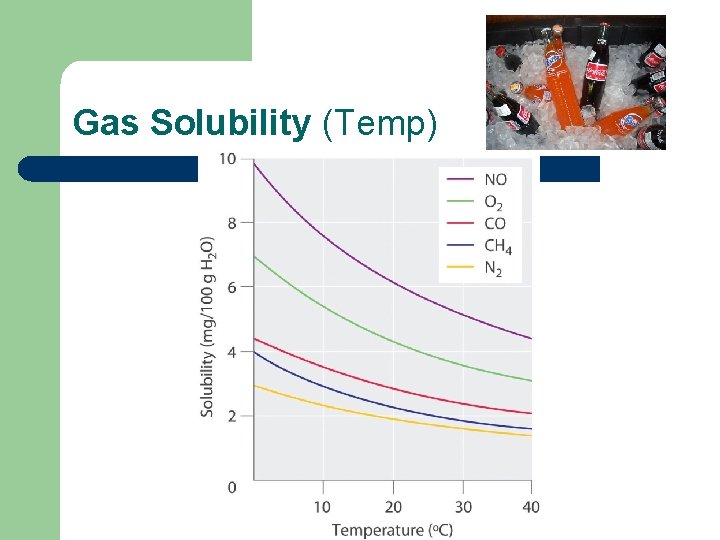
Factors that affect solubility l Temperature: – Solids l – Direct relationship; as temperature goes up solubility goes up Gases l Indirect relationship; as temperature goes up solubility goes down

Temperature vs Solubility

Temperature vs Solubility

Gas Solubility (Temp)

Factors Affecting the Rate of Dissolution (dissolving) l Stirring l Temperature l Surface Area

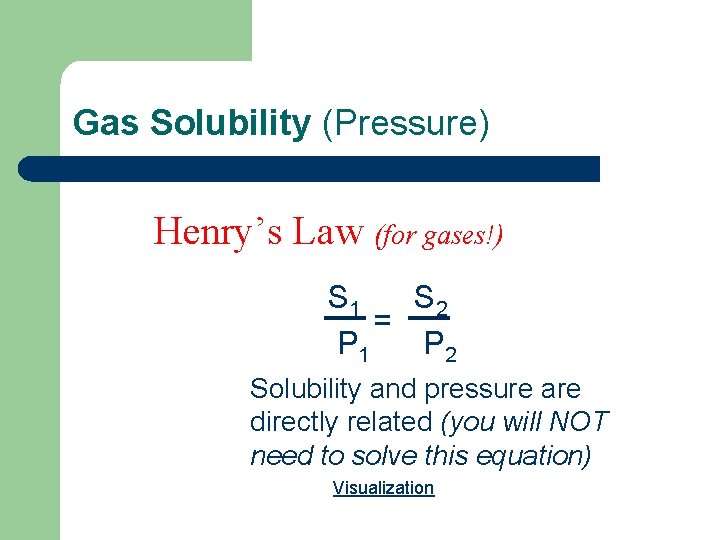
Gas Solubility (Pressure) Henry’s Law (for gases!) S 1 S 2 = P 1 P 2 Solubility and pressure are directly related (you will NOT need to solve this equation) Visualization

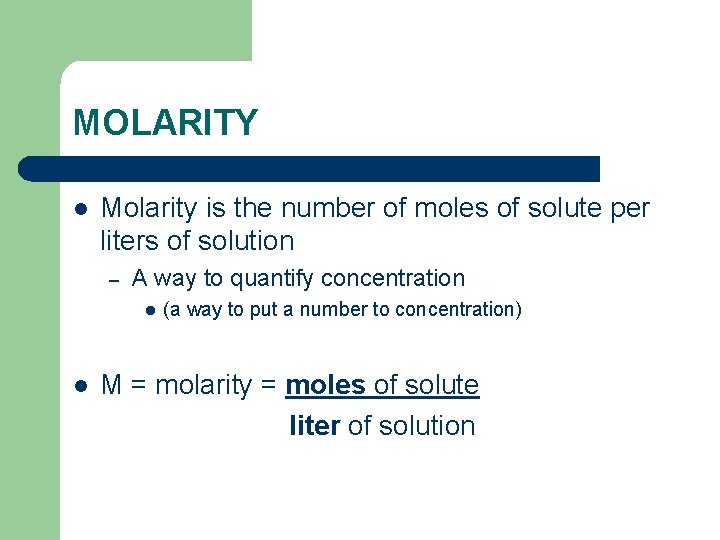
MOLARITY l Molarity is the number of moles of solute per liters of solution – A way to quantify concentration l l (a way to put a number to concentration) M = molarity = moles of solute liter of solution

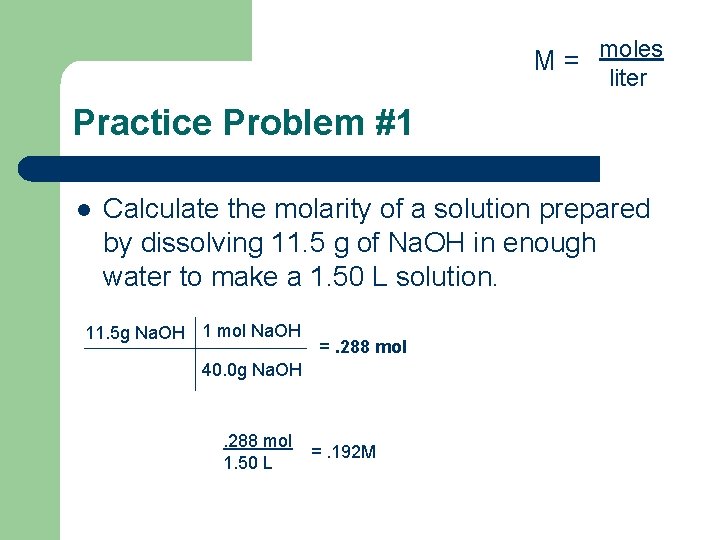
M = moles liter Practice Problem #1 l Calculate the molarity of a solution prepared by dissolving 11. 5 g of Na. OH in enough water to make a 1. 50 L solution. 11. 5 g Na. OH 1 mol Na. OH =. 288 mol 40. 0 g Na. OH . 288 mol 1. 50 L =. 192 M

Practice Problem #2 l Calculate the molarity of a solution prepared by dissolving 1. 56 g of HCl into enough water to make 26. 8 ml of solution. . 0427 moles of HCl 26. 8 m. L =. 0268 L M = 1. 59 M solution

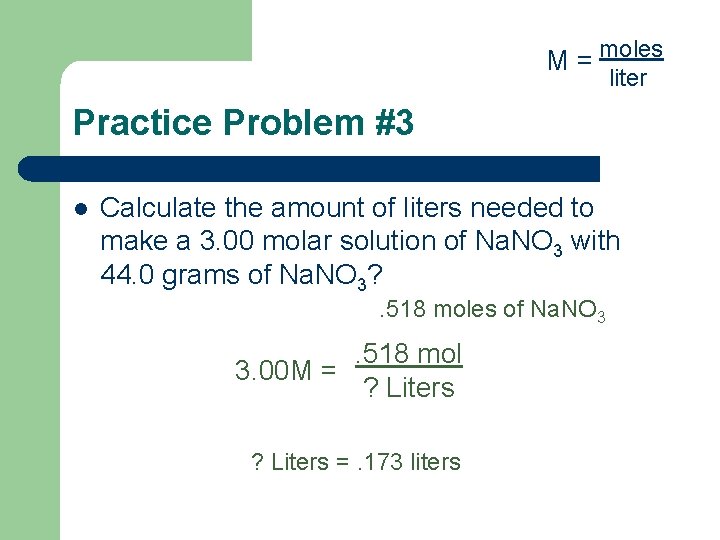
M = moles liter Practice Problem #3 l Calculate the amount of liters needed to make a 3. 00 molar solution of Na. NO 3 with 44. 0 grams of Na. NO 3? . 518 moles of Na. NO 3 . 518 mol 3. 00 M = ? Liters =. 173 liters

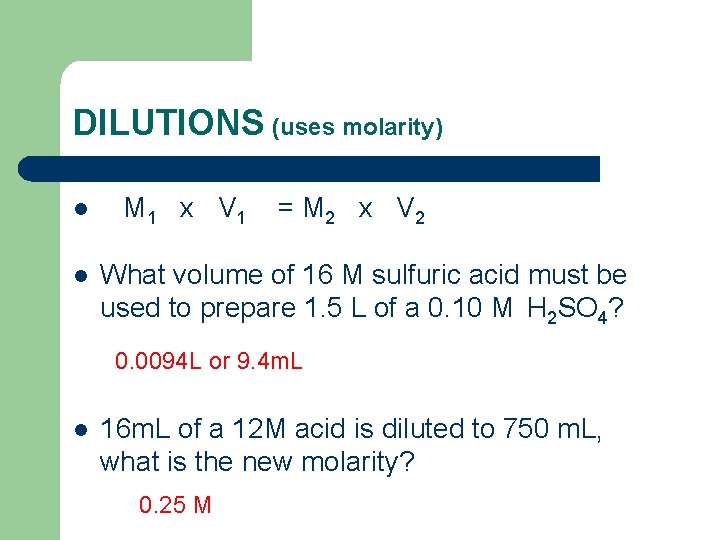
DILUTIONS (uses molarity) l l M 1 x V 1 = M 2 x V 2 What volume of 16 M sulfuric acid must be used to prepare 1. 5 L of a 0. 10 M H 2 SO 4? 0. 0094 L or 9. 4 m. L l 16 m. L of a 12 M acid is diluted to 750 m. L, what is the new molarity? 0. 25 M

M 1 x V 1 = M 2 x V 2 DILUTIONS (Real solutions) l To how much water should 25. 0 m. L of 6. 00 M HCl be added to produce a 4. 00 M solution? V 2 = 37. 5 m. L V 2 – V 1 = amount of water 37. 5 m. L – 25. 0 m. L = 12. 5 m. L of water

Freezing Point Depression in Solutions l The particles hinder the solvent from freezing by slowing the formation of solid crystals – – – This is used in winter with icy roads & sidewalks! Making Popsicles Cold Ice

Colligative Properties l A property that depends on only the number of solute particles, NOT their identity l There are 3 important colligative properties: 1. 2. 3. Boiling Point Elevation Freezing Point Depression Vapor-pressure Lowering

Molality (NOT molarity) l WE WILL NOT BE CALCULATING MOLALITY OR COLLIGATIVE PROPERTIES Similar to molarity in that it shows concentration… – Molality (m) = moles of solute Kilogram of solvent

WE WILL NOT BE CALCULATING MOLALITY OR COLLIGATIVE PROPERTIES Molality Practice (mol/kg) l 2. 4 moles of sucrose are dissolved in 320 m. L of water. What is the molality? 2. 4 moles sucrose. 320 Kg water = 7. 5 m

WE WILL NOT BE CALCULATING MOLALITY OR COLLIGATIVE PROPERTIES Molality Practice (mol/kg) l 56. 8 g of Na. Cl is dissolve in 560 m. L of water. What is the molality? . 97 mol/. 560 kg = 1. 73 m

Colligative Properties WE WILL NOT BE CALCULATING MOLALITY OR COLLIGATIVE PROPERTIES Freezing Point Depression/ Boiling Point Elevation Tf = m x kf x i OR Tb = m x kb x i m = molality i = number of ions dissolved per molecule kf = freezing point constant (given) kb = boiling point constant (given) l kf = 1. 86 C Kg/mol (for water) kb = 0. 51 C Kg/mol (for water)

WE WILL NOT BE CALCULATING MOLALITY OR COLLIGATIVE PROPERTIES Colligative Practice Problem 56. 8 g of Na. Cl is dissolved in 560 m. L of distilled water. What is the freezing point of this solution? Tf = m x kf x i l l l Step 1 -> determine m (molality)=moles/Kg Step 2 -> Plug into equation with kf (given) and i (number of ions, in this case i = 2) Step 3 -> solve for Tf Step 4 -> adjust original freezing point accordingly

WE WILL NOT BE CALCULATING MOLALITY OR COLLIGATIVE PROPERTIES Colligative Practice Problem m = 1. 73 l Tf = m x kf x i Tf = (1. 73 m) x 1. 86 C Kg/mol x 2 Tf = 6. 4 What is new freezing point? -6. 4 C

WE WILL NOT BE CALCULATING MOLALITY OR COLLIGATIVE PROPERTIES Colligative Problem: finding molar mass l A solution of a nonelectrolyte (does not dissociate in water) contains 30. 0 g of solute dissolved in 250. 0 g of water. The boiling point of the water is observed to be 101. 04°C. What is the molar mass of this substance?

A solution of a nonelectrolyte (does not dissociate in water) contains 30. 00 g of solute dissolved in 250. 0 g of water. The boiling point of the water is observed to be 101. 04°C. What is the molar mass of this substance? l Game plan: – Molar mass (of solute) units grams/mole l We are given grams of solute; must solve for moles. – We are given BP of water; we must be using Tb = m x kb x i – Are there moles anywhere in the equation? l m = moles of solute kg of solvent

WE WILL NOT BE A solution of a nonelectrolyte (does not dissociate in CALCULATING MOLALITY OR g of water) contains 30. 00 g of solute dissolved in 250. 0 COLLIGATIVE water. The boiling point of the water is observed to be PROPERTIES 101. 04°C. What is the molar mass of this substance? Tb = m x kb x i l l Tb = 101. 04°C - 100°C = 1. 04 °C m = ? moles of solute/. 2500 Kg of water kb (always given)= 0. 51 C Kg/mol i = 1 (for all nonelectrolytes 1. 04 °C = ? moles x. 51 C Kg/mol x 1. 2500 kg

WE WILL NOT BE A solution of a nonelectrolyte (does not dissociate in CALCULATING MOLALITY OR g of water) contains 30. 00 g of solute dissolved in 250. 0 COLLIGATIVE water. The boiling point of the water is observed to be PROPERTIES 101. 04°C. What is the molar mass of this substance? l ? Moles = 0. 5098 moles l Molar mass = grams/moles 30. 00 g of solute = 58. 8 g/mol 0. 5098 moles l

Solution Stoichiometry 1. How many grams of silver chromate will precipitate when 150. m. L of 0. 500 M silver nitrate are added to 100. m. L of 0. 400 M potassium chromate? 2 Ag. NO 3(aq) + K 2 Cr. O 4(aq) Ag 2 Cr. O 4(s) + 2 KNO 3(aq)

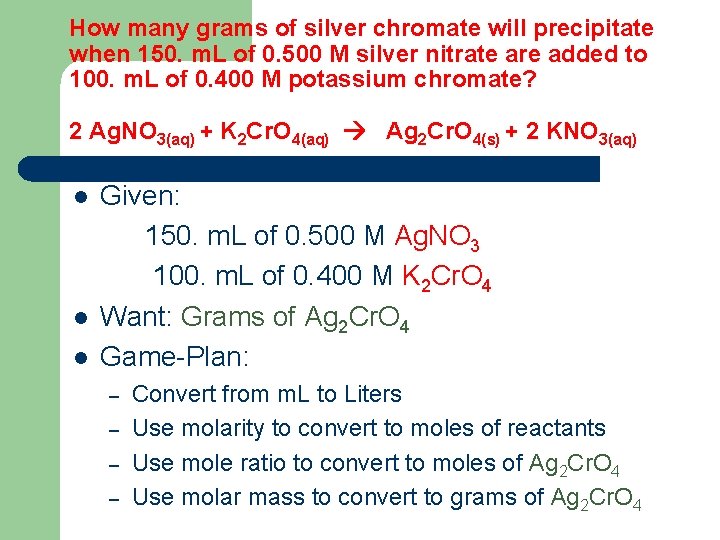
How many grams of silver chromate will precipitate when 150. m. L of 0. 500 M silver nitrate are added to 100. m. L of 0. 400 M potassium chromate? 2 Ag. NO 3(aq) + K 2 Cr. O 4(aq) Ag 2 Cr. O 4(s) + 2 KNO 3(aq) l l l Given: 150. m. L of 0. 500 M Ag. NO 3 100. m. L of 0. 400 M K 2 Cr. O 4 Want: Grams of Ag 2 Cr. O 4 Game-Plan: – – Convert from m. L to Liters Use molarity to convert to moles of reactants Use mole ratio to convert to moles of Ag 2 Cr. O 4 Use molar mass to convert to grams of Ag 2 Cr. O 4

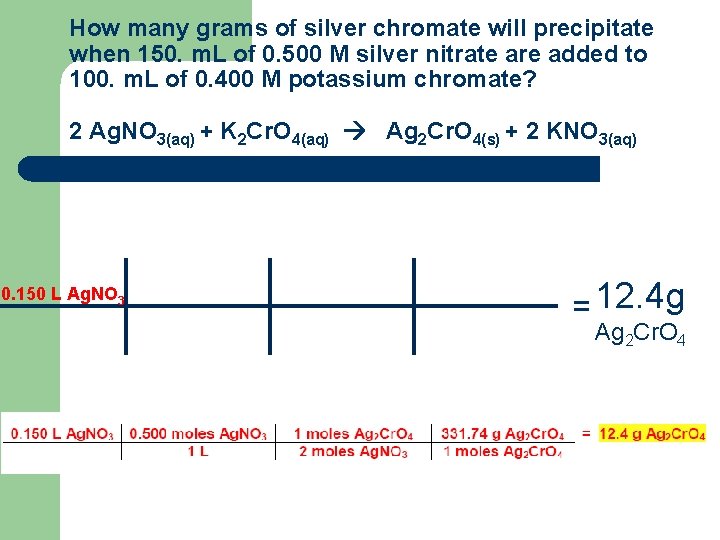
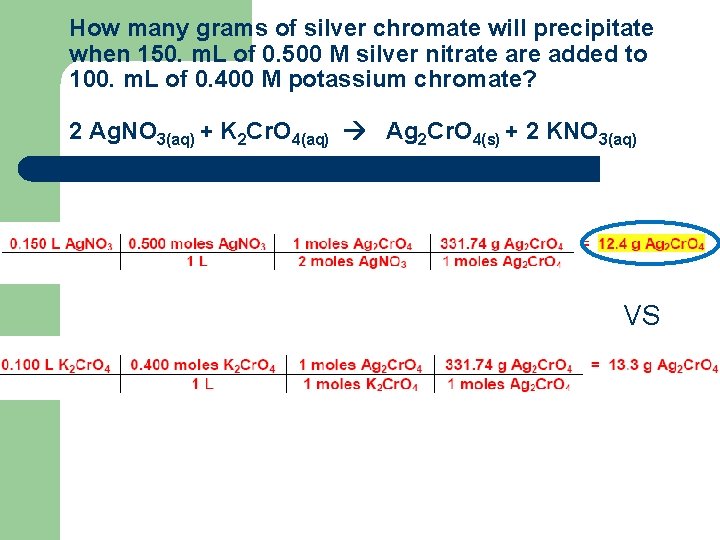
How many grams of silver chromate will precipitate when 150. m. L of 0. 500 M silver nitrate are added to 100. m. L of 0. 400 M potassium chromate? 2 Ag. NO 3(aq) + K 2 Cr. O 4(aq) Ag 2 Cr. O 4(s) + 2 KNO 3(aq) 0. 150 L Ag. NO 3 = 12. 4 g Ag 2 Cr. O 4

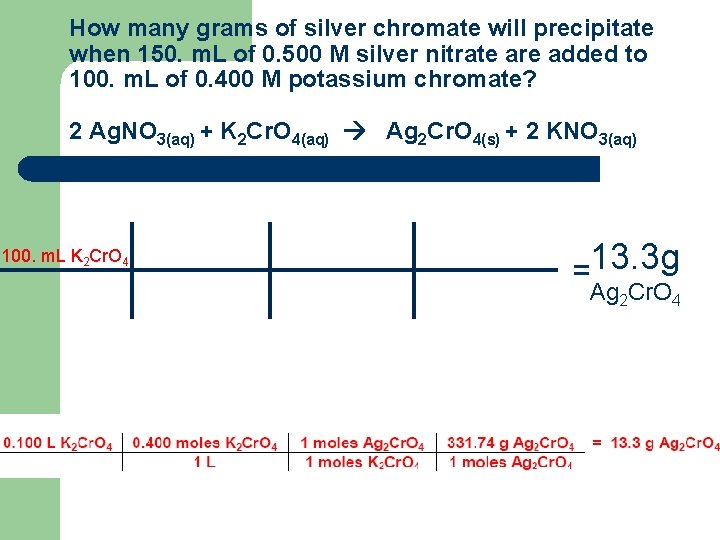
How many grams of silver chromate will precipitate when 150. m. L of 0. 500 M silver nitrate are added to 100. m. L of 0. 400 M potassium chromate? 2 Ag. NO 3(aq) + K 2 Cr. O 4(aq) Ag 2 Cr. O 4(s) + 2 KNO 3(aq) 100. m. L K 2 Cr. O 4 =13. 3 g Ag 2 Cr. O 4

How many grams of silver chromate will precipitate when 150. m. L of 0. 500 M silver nitrate are added to 100. m. L of 0. 400 M potassium chromate? 2 Ag. NO 3(aq) + K 2 Cr. O 4(aq) Ag 2 Cr. O 4(s) + 2 KNO 3(aq) VS