AP Chemistry Solutions solution homogeneous mixture solid liquid











- Slides: 35

AP Chemistry – Solutions

solution: homogeneous mixture; solid, liquid, or gas solvent: substance present in the greatest amount solute: also present; there might be more than one

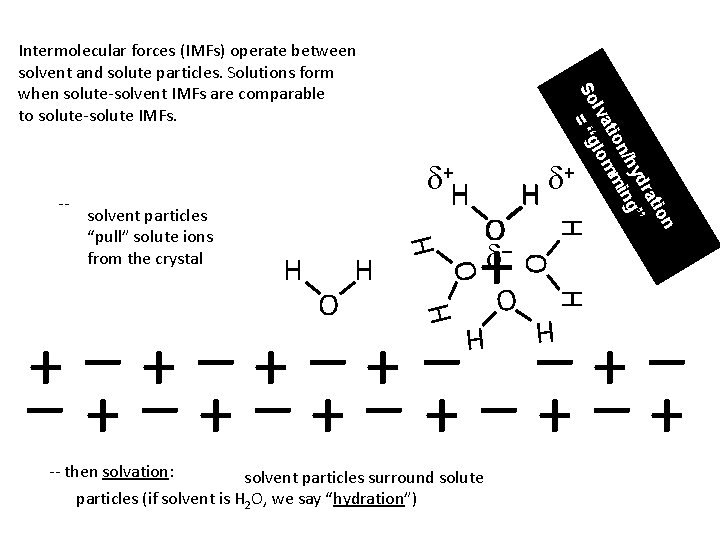
-- O d– + d H H solvent particles “pull” solute ions from the crystal d+H ion rat yd g” n/h min tio lva lom So = “g Intermolecular forces (IMFs) operate between solvent and solute particles. Solutions form when solute-solvent IMFs are comparable to solute-solute IMFs. O H H solvent particles surround solute particles (if solvent is H 2 O, we say “hydration”) H -- then solvation: O H O +–+–+–+–+ H

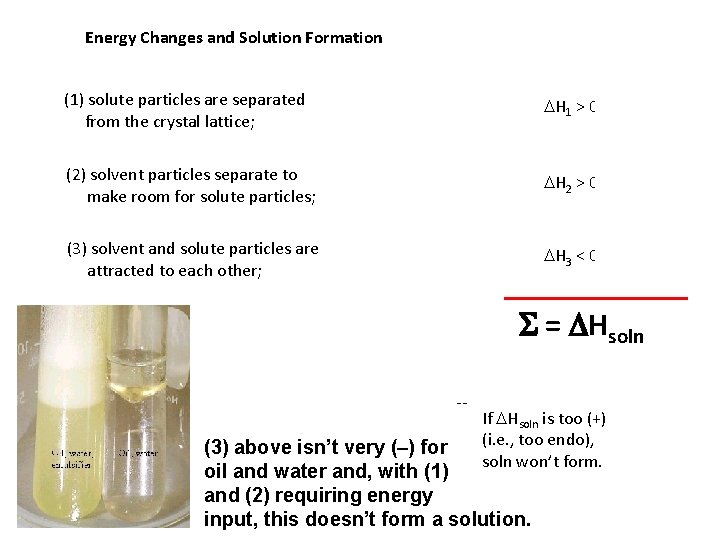
Energy Changes and Solution Formation (1) solute particles are separated from the crystal lattice; DH 1 > 0 (2) solvent particles separate to make room for solute particles; DH 2 > 0 (3) solvent and solute particles are attracted to each other; DH 3 < 0 S = DHsoln -- If DHsoln is too (+) (i. e. , too endo), soln won’t form. (3) above isn’t very (–) for oil and water and, with (1) and (2) requiring energy input, this doesn’t form a solution.

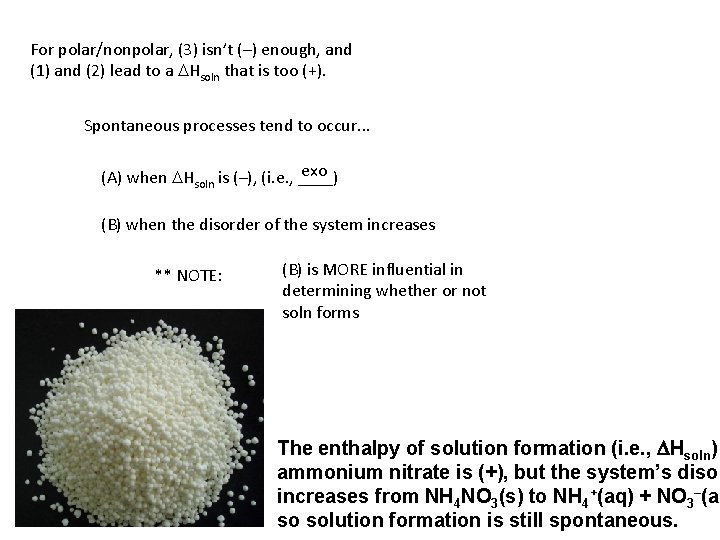
For polar/nonpolar, (3) isn’t (–) enough, and (1) and (2) lead to a DHsoln that is too (+). Spontaneous processes tend to occur. . . exo (A) when DHsoln is (–), (i. e. , ____) (B) when the disorder of the system increases ** NOTE: (B) is MORE influential in determining whether or not soln forms The enthalpy of solution formation (i. e. , DHsoln) ammonium nitrate is (+), but the system’s disor increases from NH 4 NO 3(s) to NH 4+(aq) + NO 3–(aq so solution formation is still spontaneous.

Sometimes it is not readily apparent whether a solution has been formed or whether a chemical reaction took place. To help you decide, consider the following: If the product is evaporated to dryness, a solution would give you what you started with. e. g. , Na. Cl(s) + H 2 O(l) Na. Cl(aq) Ni(s) + 2 HCl(aq) Ni. Cl 2(aq) + H 2(g) (SOLN) (RXN)

Solubility Crystallization is the opposite of the solution process. SOLUTE + SOLVENT SOLUTION crystallization -- When the rates of solution and crystallization are equal, _____ equilibrium is established. A saturated solution of Na. Cl, in which the rates of solution and crystallization are equal. solution

solubility: the amount of solute needed to form a saturated solution in a given quantity of solvent under given conditions of T and P saturated: soln is in eq. w/undissolved solute i. e. , there is solid at the bottom unsaturated: more solute could dissolve i. e. , soln is clear (MIGHT be colorless) supersaturated: the amount of dissolved solute exceeds the solubility -- soln has a clear, water-like appearance, but is VERY unstable -- addition of a seed crystal causes excess solute to crystallize, leaving a sat. soln. (w/visible solid)

A supersaturated solution crystallizing upon the addition of a seed crystal. The resulting solution is then saturated.

http: //www. youtube. com/watch? v=Hn. Sg 2 cl 09 PI http: //www. youtube. com/watch? feature=end screen&v=yxi 6 nx. Aqyew&NR=1

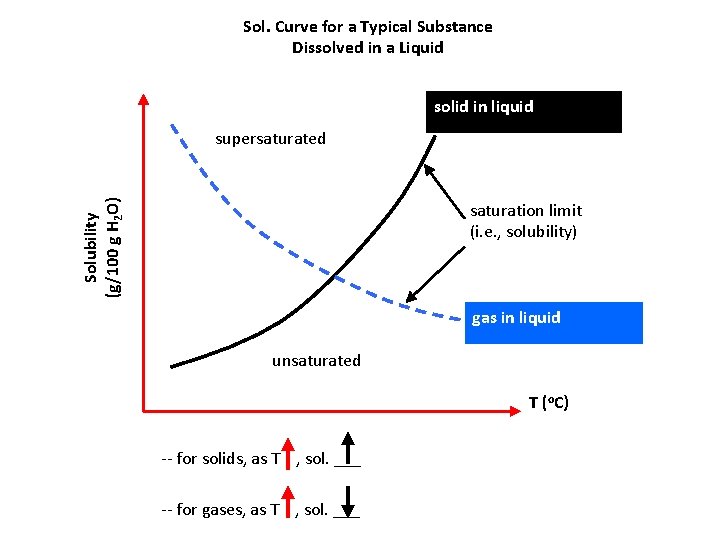
Sol. Curve for a Typical Substance Dissolved in a Liquid solid in liquid Solubility (g/100 g H 2 O) supersaturated saturation limit (i. e. , solubility) gas in liquid unsaturated T (o. C) -- for solids, as T , sol. ___ -- for gases, as T , sol. ___

Factors Affecting Solubility Solute-Solvent Interactions -- As IMFs between solute and solvent increase, increases solubility _____. miscible: describes pairs of liquids that mix in all proportions (v. immiscible) d+ d+ d+ d– d– Methanol, which is used to fuel race is miscible with water due to its high polar nature.

Low molar mass alcohols are completely miscible in water, due to H-bonding of hydroxyl group (–OH); as molar mass increases, the polarity of the alcohol molecule. . . decreases (it behaves more like a pure hydrocarbon) and miscibility decreases. e. g. , CH 3 OH vs. CH 3 CH 2 CH 2 OH -- Substances with similar IMFs tend to be soluble in one another; “like dissolves like. ” (pol/pol and np/np) -- Some network solids aren’t soluble in either polar or nonpolar solvents because of strong forces within the solid.

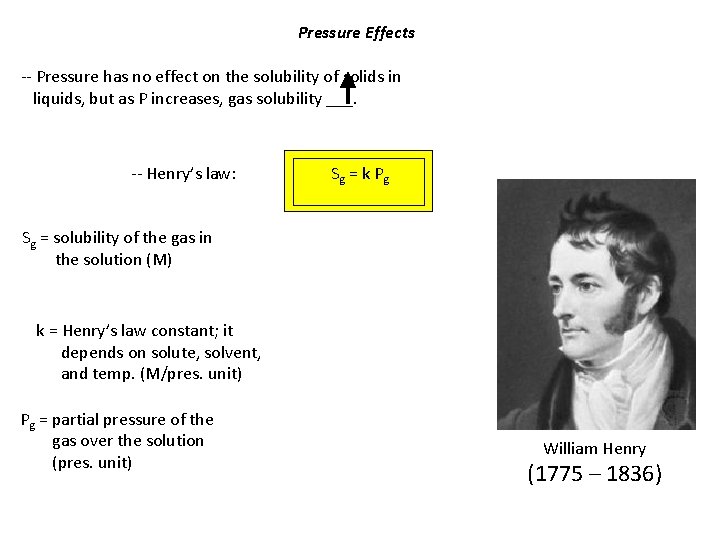
Pressure Effects -- Pressure has no effect on the solubility of solids in liquids, but as P increases, gas solubility ___. -- Henry’s law: Sg = k P g Sg = solubility of the gas in the solution (M) k = Henry’s law constant; it depends on solute, solvent, and temp. (M/pres. unit) Pg = partial pressure of the gas over the solution (pres. unit) William Henry (1775 – 1836)

A bottled soft drink at 25 o. C has CO 2 gas at a pressure of 5. 0 atm over the liquid. If the partial pressure of CO 2 in the atmosphere is 4. 0 x 10– 4 atm and the Henry’s law constant for CO 2 over water at 25 o. C is 3. 1 x 10– 2 M/atm, calculate the solubility of the CO 2 both before and after the bottle is opened. Sg = k P g BEFORE Sg = 3. 1 x 10– 2 M/atm (5. 0 atm) = 0. 16 M (fresh) AFTER Sg = 3. 1 x 10– 2 M/atm (4. 0 x 10– 4 atm) = 1. 2 x 10– 5 M (flat)

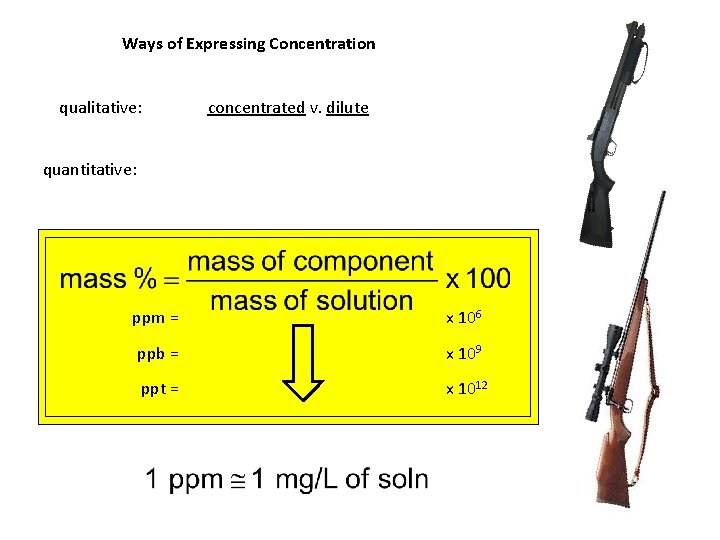
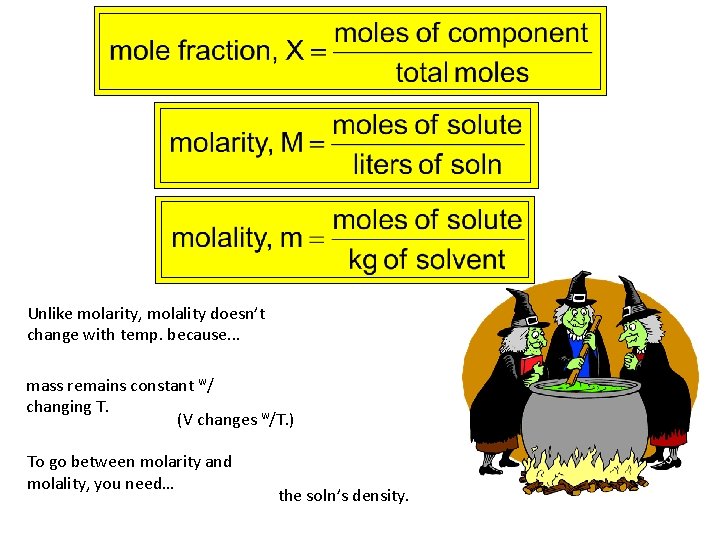
Ways of Expressing Concentration qualitative: concentrated v. dilute quantitative: ppm = x 106 ppb = x 109 ppt = x 1012

Unlike molarity, molality doesn’t change with temp. because. . . mass remains constant w/ changing T. (V changes w/T. ) To go between molarity and molality, you need… the soln’s density.

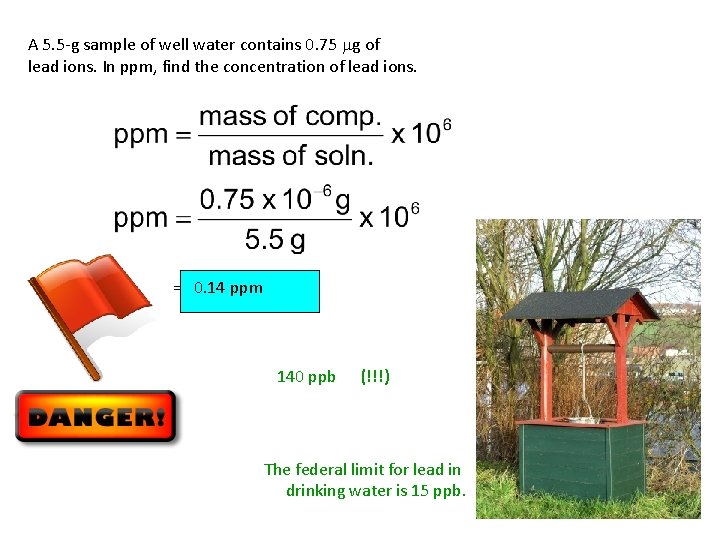
A 5. 5 -g sample of well water contains 0. 75 mg of lead ions. In ppm, find the concentration of lead ions. = 0. 14 ppm 140 ppb (!!!) The federal limit for lead in drinking water is 15 ppb.

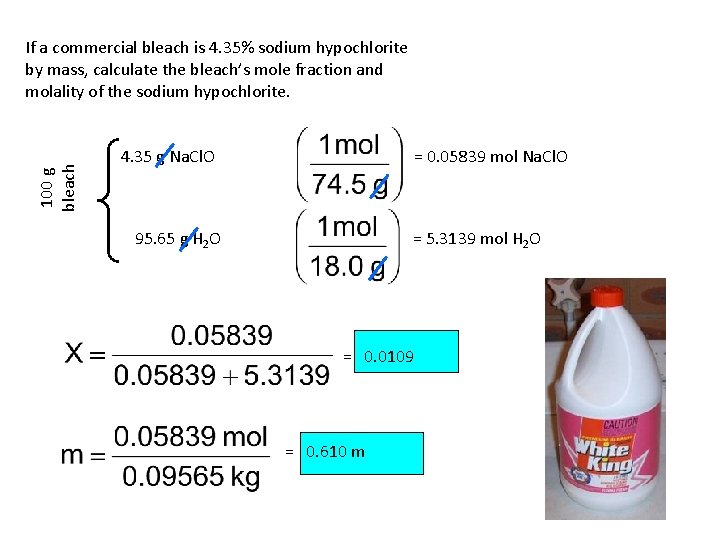
100 g bleach If a commercial bleach is 4. 35% sodium hypochlorite by mass, calculate the bleach’s mole fraction and molality of the sodium hypochlorite. 4. 35 g Na. Cl. O = 0. 05839 mol Na. Cl. O 95. 65 g H 2 O = 5. 3139 mol H 2 O = 0. 0109 = 0. 610 m

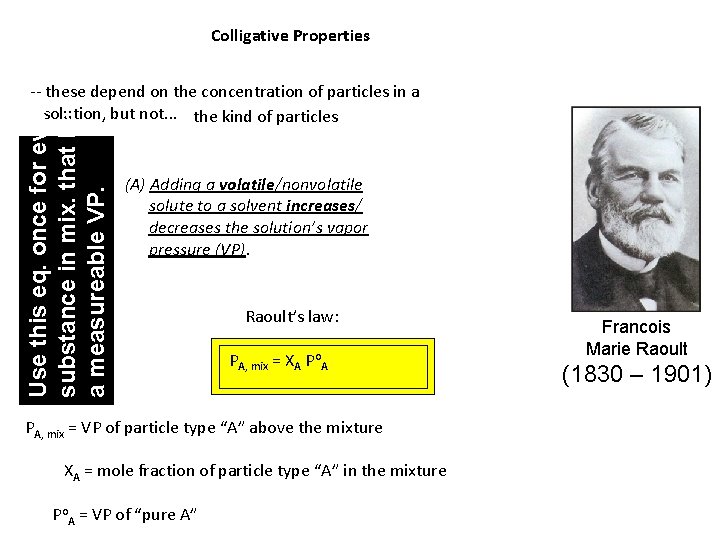
Colligative Properties Use this eq. once for every substance in mix. that has a measureable VP. -- these depend on the concentration of particles in a solution, but not. . . the kind of particles (A) Adding a volatile/nonvolatile solute to a solvent increases/ decreases the solution’s vapor pressure (VP). Raoult’s law: PA, mix = XA Po. A PA, mix = VP of particle type “A” above the mixture XA = mole fraction of particle type “A” in the mixture Po. A = VP of “pure A” Francois Marie Raoult (1830 – 1901)

Ideal solutions obey Raoult’s law. Such solutions have. . . -- a low concentration (i. e. , are relatively dilute) -- solute and solvent particles that are similar in size and have similar IMFs

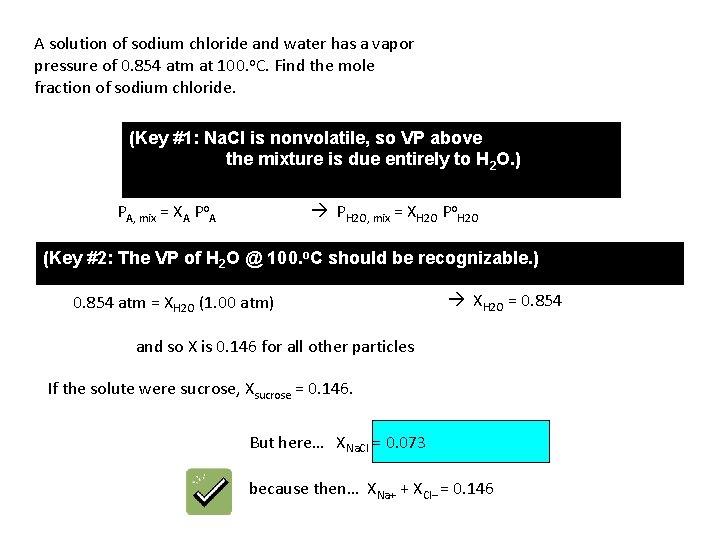
A solution of sodium chloride and water has a vapor pressure of 0. 854 atm at 100. o. C. Find the mole fraction of sodium chloride. (Key #1: Na. Cl is nonvolatile, so VP above the mixture is due entirely to H 2 O. ) PH 2 O, mix = XH 2 O Po. H 2 O PA, mix = XA Po. A (Key #2: The VP of H 2 O @ 100. o. C should be recognizable. ) 0. 854 atm = XH 2 O (1. 00 atm) XH 2 O = 0. 854 and so X is 0. 146 for all other particles If the solute were sucrose, Xsucrose = 0. 146. But here… XNa. Cl = 0. 073 because then… XNa+ + XCl– = 0. 146

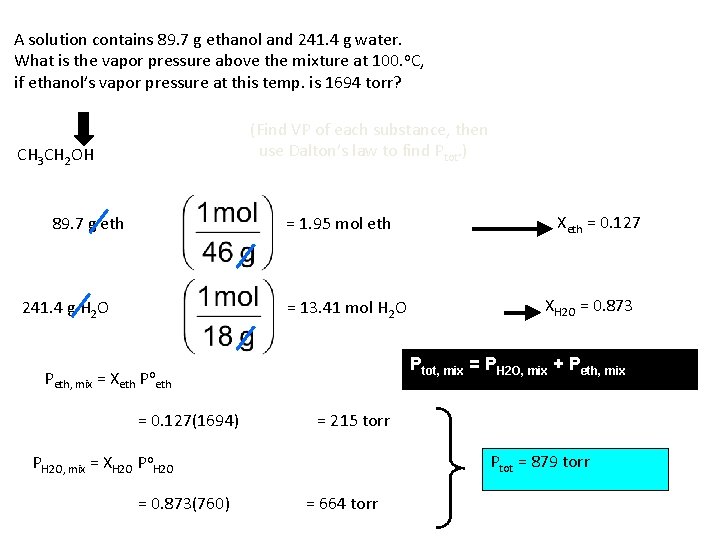
A solution contains 89. 7 g ethanol and 241. 4 g water. What is the vapor pressure above the mixture at 100. o. C, if ethanol’s vapor pressure at this temp. is 1694 torr? (Find VP of each substance, then use Dalton’s law to find Ptot. ) CH 3 CH 2 OH 89. 7 g eth = 1. 95 mol eth 241. 4 g H 2 O = 13. 41 mol H 2 O = 215 torr Ptot = 879 torr PH 2 O, mix = XH 2 O Po. H 2 O = 0. 873(760) XH 2 O = 0. 873 Ptot, mix = PH 2 O, mix + Peth, mix = Xeth Poeth = 0. 127(1694) Xeth = 0. 127 = 664 torr

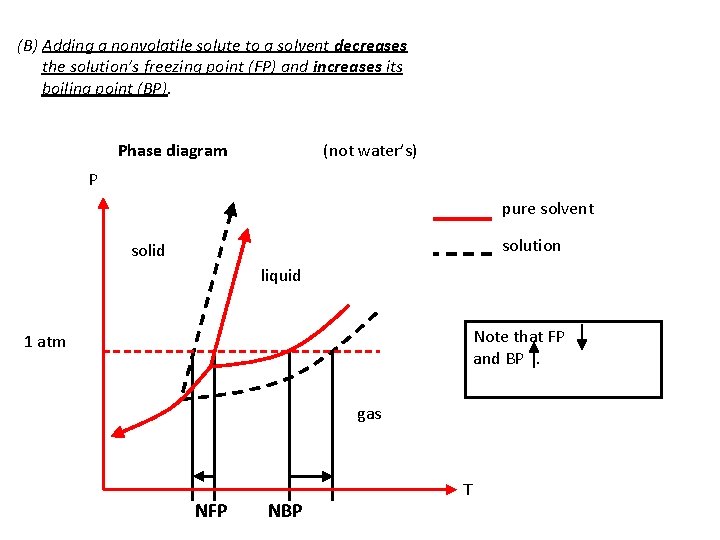
(B) Adding a nonvolatile solute to a solvent decreases the solution’s freezing point (FP) and increases its boiling point (BP). Phase diagram (not water’s) P pure solvent solution solid liquid Note that FP and BP. 1 atm gas NFP NBP T

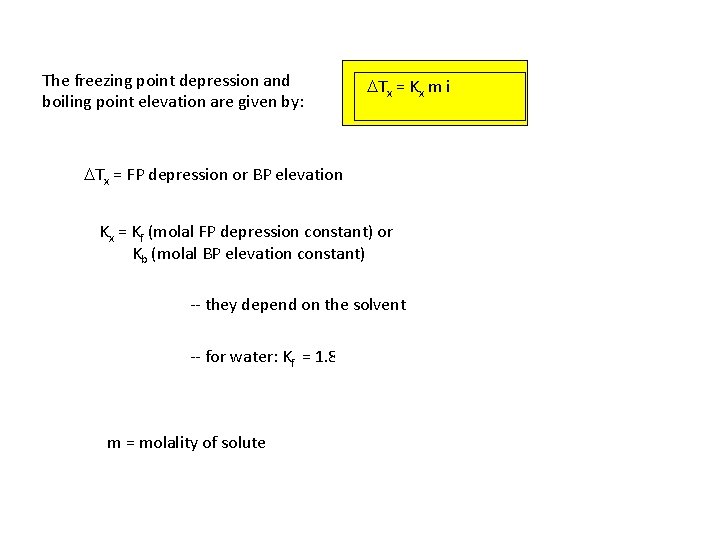
The freezing point depression and boiling point elevation are given by: DTx = Kx m i DTx = FP depression or BP elevation Kx = Kf (molal FP depression constant) or Kb (molal BP elevation constant) -- they depend on the solvent -- for water: Kf = 1. 86 o. C/m Kb = 0. 52 o. C/m m = molality of solute

i = van’t Hoff factor (accounts for # of particles in solution) In aq. soln. , assume that… 1 for nonelectrolytes -- i = __ 2 for KBr, Na. Cl, etc. -- i = __ 3 for Ca. Cl , etc. -- i = __ 2 In reality, the van’t Hoff factor isn’t always an integer. Use the guidelines unless given information to the contrary. Jacobus Henricus van’t Hof (1852 – 1911)

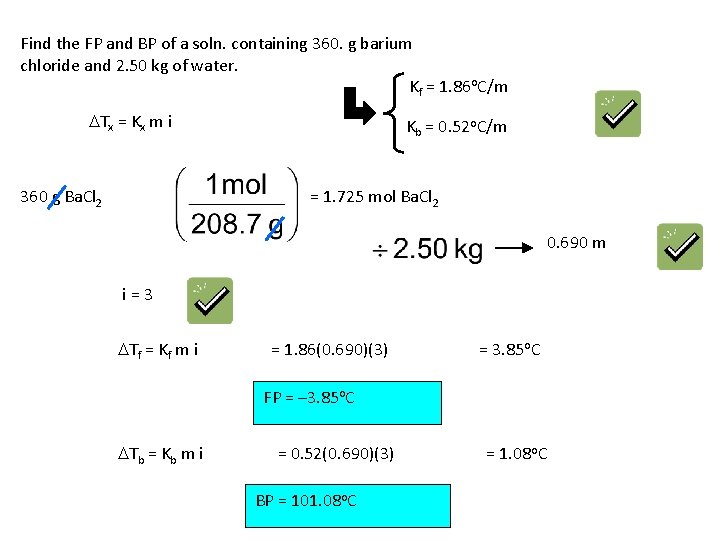
Find the FP and BP of a soln. containing 360. g barium chloride and 2. 50 kg of water. Kf = 1. 86 o. C/m DTx = Kx m i 360 g Ba. Cl 2 Kb = 0. 52 o. C/m = 1. 725 mol Ba. Cl 2 0. 690 m i=3 DTf = Kf m i = 1. 86(0. 690)(3) = 3. 85 o. C FP = – 3. 85 o. C DTb = Kb m i = 0. 52(0. 690)(3) BP = 101. 08 o. C = 1. 08 o. C

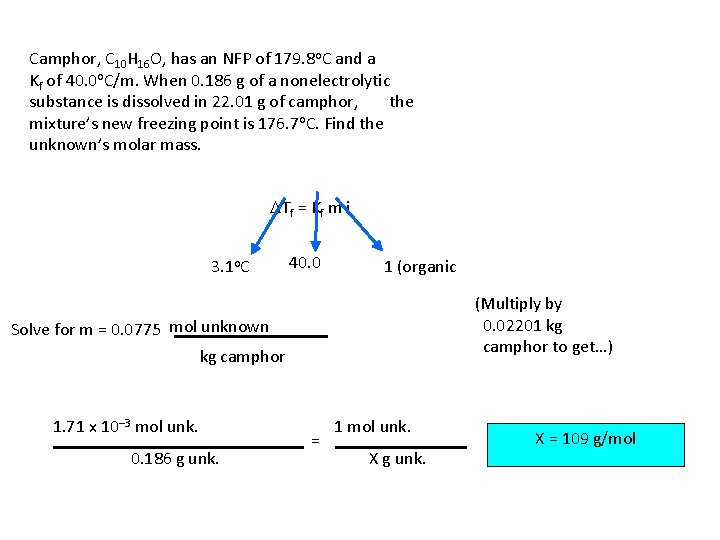
Camphor, C 10 H 16 O, has an NFP of 179. 8 o. C and a Kf of 40. 0 o. C/m. When 0. 186 g of a nonelectrolytic substance is dissolved in 22. 01 g of camphor, the mixture’s new freezing point is 176. 7 o. C. Find the unknown’s molar mass. DTf = Kf m i 3. 1 o. C 40. 0 1 (organic = nonelec. ) (Multiply by 0. 02201 kg camphor to get…) Solve for m = 0. 0775 mol unknown kg camphor 1. 71 x 10– 3 mol unk. 0. 186 g unk. = 1 mol unk. X g unk. X = 109 g/mol

(C) Adding a nonvolatile solute to a solvent increases the solution’s osmotic pressure. more conc. (in terms of solute) = higher osmotic pres. Osmosis is the net movement of solvent away from a soln. w/a lower solute [ ] toward a soln. w/a higher solute [ ]. Another way to say this is that osmosis is the diffusion of a solvent – i. e. , from an area of higher solvent [ ] to an area of lower solvent [ ] – through a semipermeable membrane. Dialysis tubing used with various sugar solns.

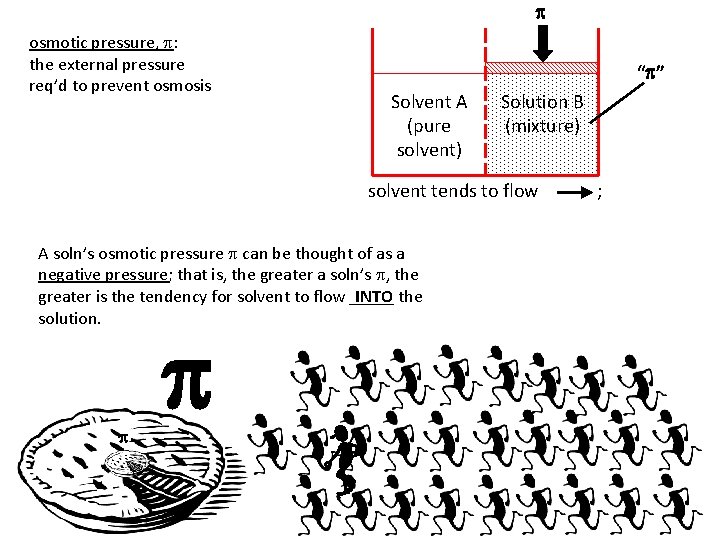
p osmotic pressure, p: the external pressure req’d to prevent osmosis “p” Solvent A (pure solvent) Solution B (mixture) solvent tends to flow ; application of p. . . prevents flow A soln’s osmotic pressure p can be thought of as a negative pressure; that is, the greater a soln’s p, the greater is the tendency for solvent to flow _____ INTO the solution. p p

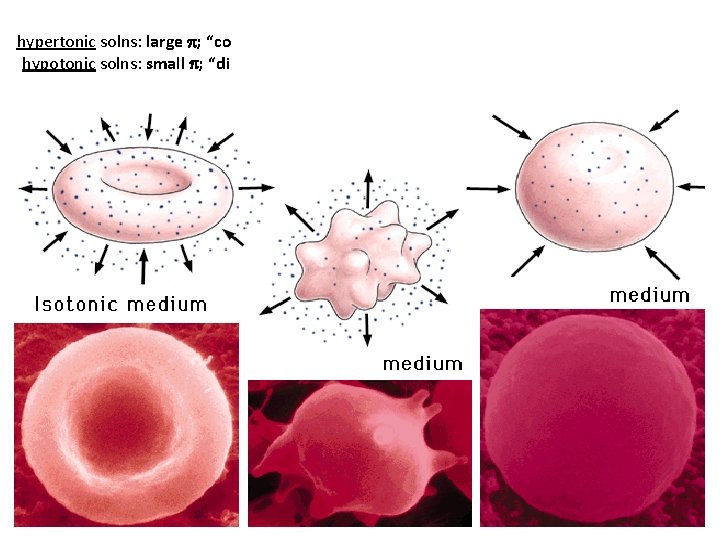
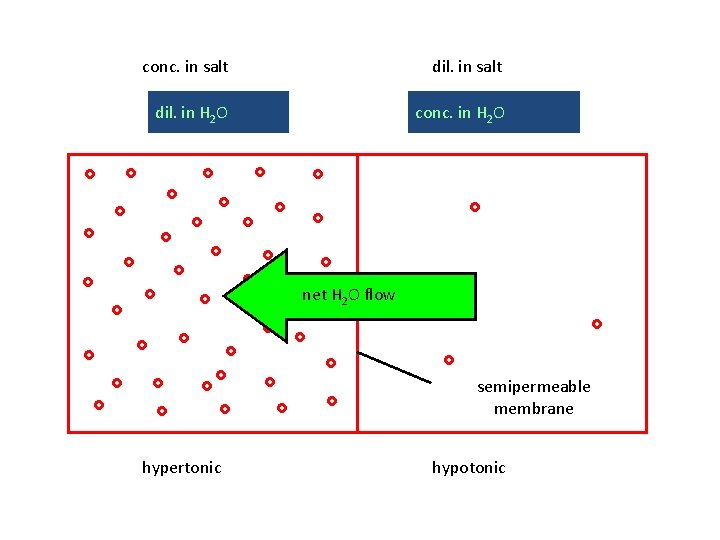
hypertonic solns: large p; “conc. ” (in terms of solute) hypotonic solns: small p; “dilute” (in terms of solute)

conc. in salt dil. in H 2 O conc. in H 2 O net H 2 O flow semipermeable membrane hypertonic hypotonic

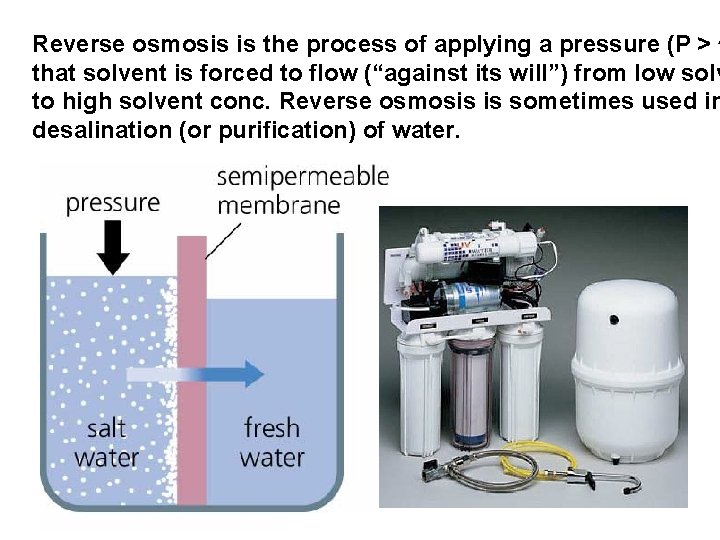
Reverse osmosis is the process of applying a pressure (P > p that solvent is forced to flow (“against its will”) from low solv to high solvent conc. Reverse osmosis is sometimes used in desalination (or purification) of water.

osmotic pressure equation: p. V=n. RTi n = # of moles of particles V = solution volume, in L R = 8. 314 L-k. Pa/mol-K = 0. 08206 L-atm/mol-K T = absolute temp. (i. e. , in K) i = van’t Hoff factor An early form of the osmotic pressure equation was proposed by van’t Hoff. The equation was improved to its current form by Harmon Northrop Morse, an American chemist who lived from 1848 to 1920.

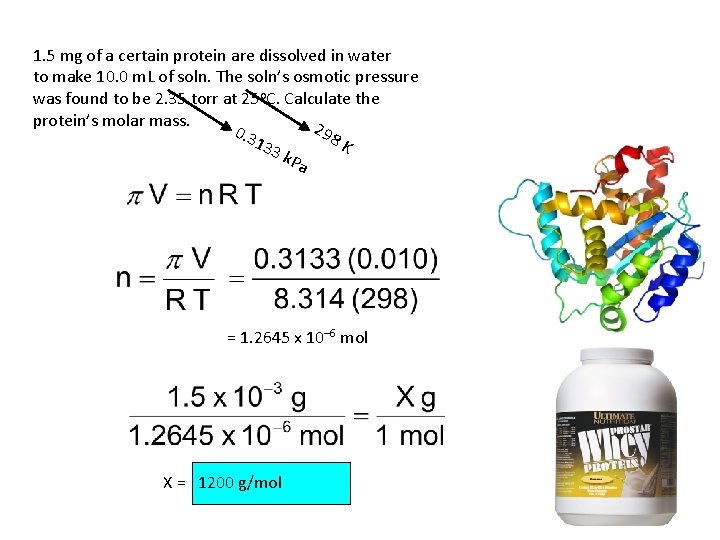
1. 5 mg of a certain protein are dissolved in water to make 10. 0 m. L of soln. The soln’s osmotic pressure was found to be 2. 35 torr at 25 o. C. Calculate the protein’s molar mass. 29 0. 3 8 K 133 k. Pa = 1. 2645 x 10– 6 mol X = 1200 g/mol