Solutions Solutions What are solutions a homogeneous mixture

























- Slides: 34

Solutions

Solutions What are solutions? Ø a homogeneous mixture of two or more substances; the different substances cannot be mechanically separated or seen. Ø A solution may be a solid (stainless steel), a liquid (iced tea) or a gas (the air we breathe). Ø Solvent: the substance that is present in the largest amount - usually determines the phase of the solution Ø Solute: the substance present in the smallest amount – what is dissolved in the solvent. Ø We will deal mainly with solutions where water is the solvent (aqueous solutions) Ø Why is water considered to be the universal solvent? Ø Water is able to dissolve many types of solutes.

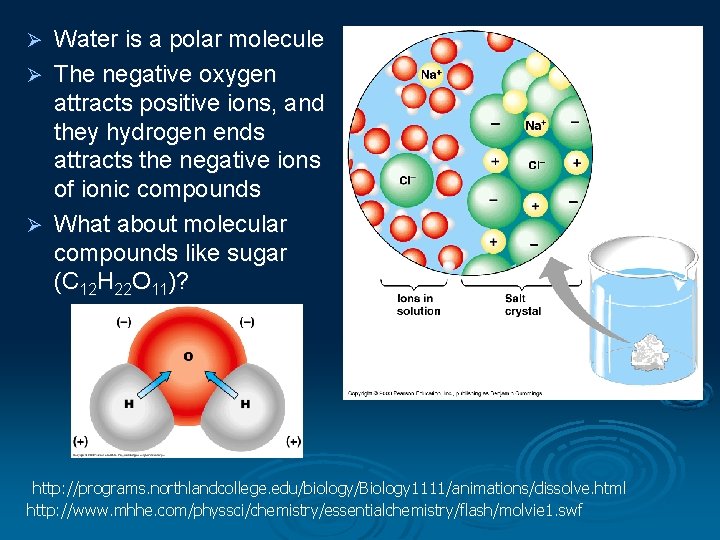
Water is a polar molecule Ø The negative oxygen attracts positive ions, and they hydrogen ends attracts the negative ions of ionic compounds Ø What about molecular compounds like sugar (C 12 H 22 O 11)? Ø http: //programs. northlandcollege. edu/biology/Biology 1111/animations/dissolve. html http: //www. mhhe. com/physsci/chemistry/essentialchemistry/flash/molvie 1. swf

Ø Electrolyte: a substance that conducts electricity when dissolved in water. l Acids, bases and soluble ionic solutions are electrolytes because they dissolve into ions. Insoluble ionic compounds are very weak electrolytes Ø Non-electrolyte: a substance that does not conduct electricity when dissolved in water. l Molecular compounds are non-electrolytes because they do not dissolve into ions

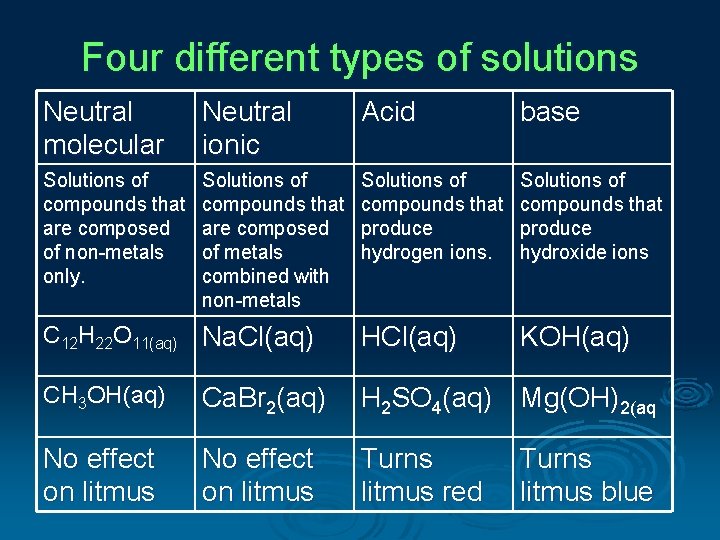
Four different types of solutions Neutral molecular Neutral ionic Acid base Solutions of compounds that are composed of non-metals only. Solutions of compounds that are composed of metals combined with non-metals Solutions of compounds that produce hydrogen ions. Solutions of compounds that produce hydroxide ions C 12 H 22 O 11(aq) Na. Cl(aq) HCl(aq) KOH(aq) CH 3 OH(aq) Ca. Br 2(aq) H 2 SO 4(aq) Mg(OH)2(aq No effect on litmus Turns litmus red Turns litmus blue

1. Neutral Molecular Ø Solutions containing molecular compounds (non metals sharing electrons) Ø No ions form in solution, therefore do NOT conduct electricity (non-electrolytes) Ø No change in color with litmus paper Ø Form colorless solutions Ø Examples: 2. Neutral Ionic Ø Dissociate in water to form positive and negative ions Ø Conduct electricity (electrolytes) Ø No change in color with litmus paper Ø Form colorless and colorful solutions Ø Examples:

3. Acidic Solutions Ø Form when an acid is dissolved in water Ø acid dissociates into positive hydrogen ions and negative ions Ø The concentration of H+ ions in solution indicate the p. H (strength of acid) Ø Electrolytes (conduct electricity) Ø Turn blue litmus paper red 4. Basic Solutions Ø Bases - presence hydroxide ions (OH-) Ø Electrolytes Ø Turn red litmus paper blue Ø Conc. of OH- ions indicates p. H level Ø Examples


Acids and Bases Defined Acids are empirically defined as substances that: Ø Taste sour Ø Conduct electricity (they are electrolytes) Ø React with metals to produce hydrogen gas Ø Turn blue litmus paper red Ø Their p. H ranges between 0 and 7 Bases are empirically defined as substances that: Ø Taste bitter Ø Conduct electricity (they are electrolytes) Ø Feel soapy or slippery Ø Turn red litmus paper blue Ø Their p. H ranges between 7 and 14

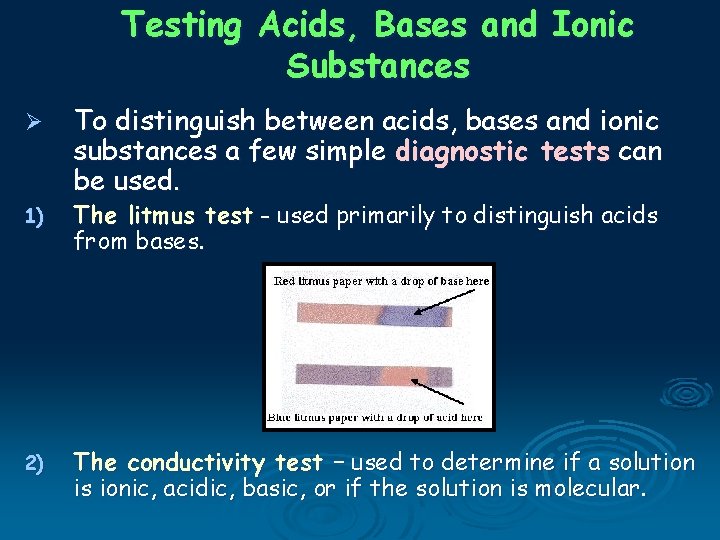
Testing Acids, Bases and Ionic Substances Ø To distinguish between acids, bases and ionic substances a few simple diagnostic tests can be used. 1) The litmus test - used primarily to distinguish acids from bases. 2) The conductivity test – used to determine if a solution is ionic, acidic, basic, or if the solution is molecular.

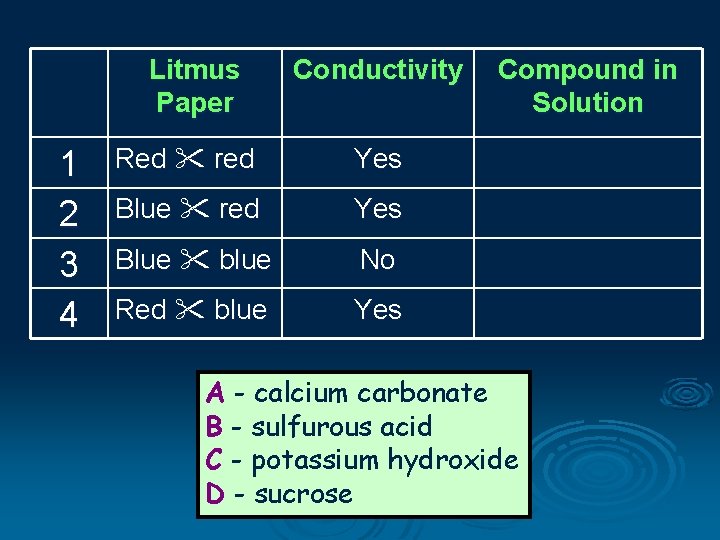
Litmus Paper 1 2 3 4 Conductivity Red red Yes Blue blue No Red blue Yes Compound in Solution A - calcium carbonate B - sulfurous acid C - potassium hydroxide D - sucrose

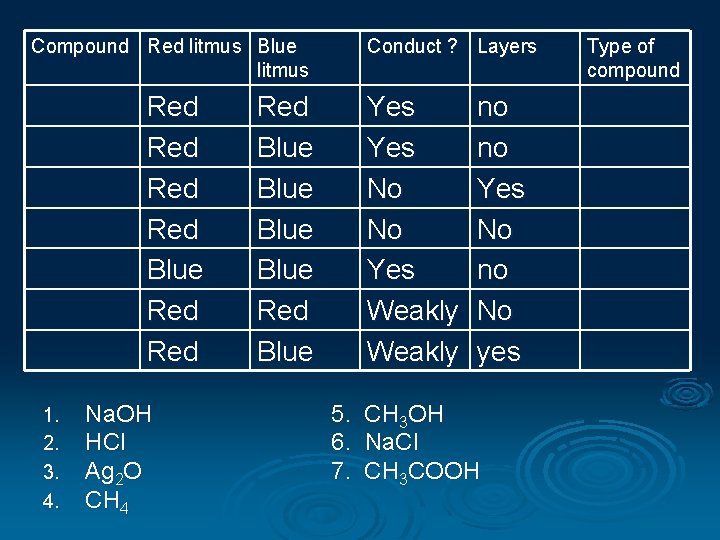
Compound Red litmus Blue litmus Red Red Blue Red 1. 2. 3. 4. Na. OH HCl Ag 2 O CH 4 Conduct ? Layers Red Blue Red Blue Yes No No Yes Weakly 5. 6. 7. no no Yes No no No yes CH 3 OH Na. Cl CH 3 COOH Type of compound

Substances Present in Water Ø Solutions made by dissolving solutes in water are called aqueous solutions. Ø If the temperature of the solution rises as a solute is dissolved, the reaction is said to be exothermic; energy is released to the solvent * energy is a product in the chemical reaction. Ø If the temperature of the solution decreases as a solute is dissolved, the reaction is said to be endothermic; energy is absorbed in the solvent. * energy is a reactant in the chemical reaction.

Dissociation, Ionization & Separation We use our solubility table to determine if something dissociates or not Ø Dissociation: the separation of ions from an ionic compound when it dissolves in water. Ø l Ex. If magnesium chloride is dissolved in water, it will form magnesium ions and chloride ions. Mg(Cl)2(s) Mg 2+(aq) + 2 Cl-(aq) Ø Please note that the equation must be balanced the same as any other equation. Also note that the charges on both sides of the equation are balanced. l Ex. K 2 SO 4(s) 2 K+(aq) + SO 42 -(aq) Ca(NO 3)2(s) Ca 2+(aq) + 2 NO 3 -(aq)

Ø If the ionic compound does not dissolve in water, it will remain as an ionic compound in water. l Ø Molecular compounds do not dissociate into respective ions in water. They dissolve in solution. l Ø Ex. Ag. Br(s) Ex. C 6 H 12 O 6(s) C 6 H 12 O 6(aq) The formation of charged ions from neutral atoms by the action of the solvent is called ionization. l Acids undergo ionization in aqueous solution. • When acids are not dissolved in water, they are molecular compounds (two nonmetals). • When acids are dissolved in water they conduct electricity. Therefore, we assume that they ionize in solution; they form ions once they have dissolved in water. • Ex. HCl(g) H+(aq) + Cl-(aq)

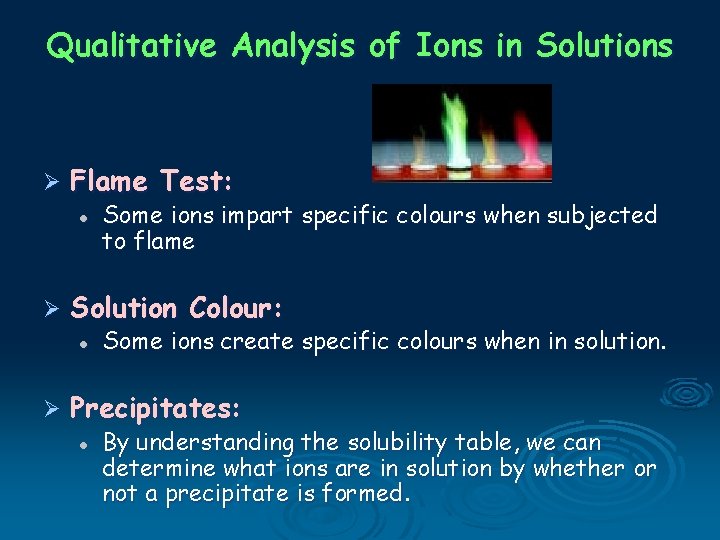
Qualitative Analysis of Ions in Solutions Ø Flame Test: l Ø Solution Colour: l Ø Some ions impart specific colours when subjected to flame Some ions create specific colours when in solution. Precipitates: l By understanding the solubility table, we can determine what ions are in solution by whether or not a precipitate is formed.


Concentration There are many ways to measure concentration. 3 common ways: 1) Percentage of solute compared to solvent by volume (%v/v) 2) Moles of solute per unit volume of solution (mol/L). The units for concentration are usually written as mol/L. 3) You will also see ppm – parts per million (mg/L).

Ways to Write Concentration Using Percents Ø We can use two methods of representing concentrations based on percent Ø % by mass (%m/v) or % by volume (%v/v) Ø Simply put the solute is put in a ratio with 100 ml (because percent is out of 100) of solution Ø We will focus on (%v/v)

(%v/v) Examples 45 ml of methyl alcohol is mixed with 55 ml of water. What is the % v/v of the solution Ø 45 is the solute, (45 + 55) is the total solution Ø 45 ml / 100 ml is 45% Ø how many grams of Na. Cl must be added to 25. 0 ml of water to prepare a 7. 50% solution Ø 7. 5 / 100 = X / 25 =1. 875 g Ø

PPM one way of representing solution concentration is ppm (parts per million) Ø simply put its mg / L Ø 8 mg of solute in 1 L of solution is 8 ppm Ø Example: some water has 6. 3 X 10 -3 g of lead per 375 m. L of solution. What is the ppm concentration? Ø 6. 3 X 10 -3 g / 375 m. L convert g to mg and m. L to L so 6. 3 mg /. 375 L = X / 1 L =16. 8 ppm Ø

Concentration in Solution Several ways to indicate the strength or concentration of a solution. Ø Generally expressed as the number of moles of solute dissolved in one liter of solution. Ø Concentration is often referred to as molarity (M). Ø The symbol for concentration is C. Ø Ø To calculate the concentration of a solution, use the following formula: C = n V C = concentration (mol/L) n = number of moles (mol) V = volume (L)

Molar Concentration Examples Ø What is the concentration made from dissolving 8. 0 moles of Cu. SO 4 in 2. 2 litres of water Ø Step 1 l l Write down what you know and don’t know n = 8. 0 mols M = 159. 62 g/mol v = 2. 2 L

Ø Step 2 l l Write down and rearrange your formula c=n/v Ø Step 3 l l l Plug in your number and include your units C = (8. 0 mols) / 2. 2 L = 3. 63636 mol/L SIG DIGS = 3. 6 mol/L

More Examples: Ø Example 2: What is the concentration when 5. 2 moles of hydrosulfuric acid are dissolved in 500 m. L of water? Step one: Convert volume to liters, mass to moles. 500 m. L = 0. 500 L Step two: Calculate concentration. C = 5. 2 mol / 0. 500 L = 10 mol/L

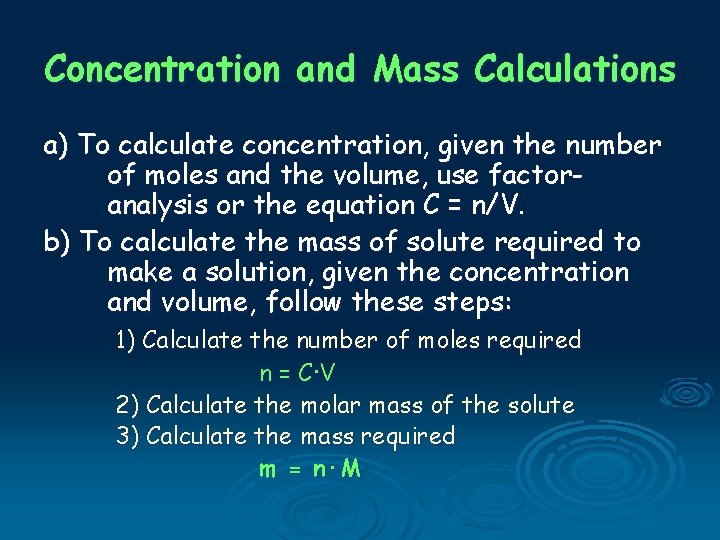
Concentration and Mass Calculations a) To calculate concentration, given the number of moles and the volume, use factoranalysis or the equation C = n/V. b) To calculate the mass of solute required to make a solution, given the concentration and volume, follow these steps: 1) Calculate the number of moles required n = C·V 2) Calculate the molar mass of the solute 3) Calculate the mass required m = n·M

Ø you may have to use both formulas we know to solve questions: m=n. M and c=n/v Ø Example: What is the mass of solute in 4. 50 L of a 2. 4 mol/L solution of HCl ?

Solution Preparation When a chemist prepares a standard solution from a solid reagent, the following steps should be taken: 1) 2) 3) 4) 5) 6) Calculate the required mass of solute. Obtain the required mass of solute in a clean, dry beaker. Dissolve the solute in distilled water using about one half of the final solution volume. Transfer the solution and all the water used to rinse the equipment into a clean volumetric flask. Add distilled water until the desired volume is reached. Stopper the flask and mix the solution by inverting the flask several times.

Dilution Calculations The process of decreasing the concentration of a solution usually by adding more solvent. Ø During a dilution procedure the addition of water changes the volume of the original solution as well as the concentration. HOWEVER the original number of moles remains the same. Ø ni (before diluting) = nf (after diluting) therefore, Ci · Vi = Cf · Vf Ci = initial concentration Cf = final concentration Vi = initial volume Vf = final volume

Dilution Problems Eg. Water is added to 200 m. L of a 2. 40 mol/L of NH 3 cleaning solution until the final volume is 1. 00 L. Find the molar concentration of the final diluted solution. Ø 0. 200 L x 2. 40 mol/L = 1. 00 L x c = 0. 480 mol/L Ø Ø Change a solution of 30 m. L of 0. 430 mol/L of KIO solution to the solutions of the following volumes: 40 m. L, 25 m. L, 180 m. L

More Examples Ø Example 3: What is the concentration of a solution if 30. 0 m. L of 4. 00 mol/L Na. Br(aq) was diluted to a new volume of 1. 50 L? Ø Example 4: What is the original concentration of a stock solution if 40 m. L of the stock solution was used to make 2. 00 L of a 0. 0100 mol/L solution?

Diluting the Solution Once a standard solution has been prepared, a chemist may wish to dilute the solution further. To do this, please follow these steps: 1) 2) 3) 4) 5) 6) Calculate the volume of standard solution required to make the diluted solution. Add about one half of the final volume of distilled water to the volumetric flask. Measure the required volume of stock solution using a pipet (see page 532 of your textbook). Transfer the stock solution to the volumetric flask. Add distilled water to the bottom of the calibration line. Stopper and invert the flask several times to mix the solution.

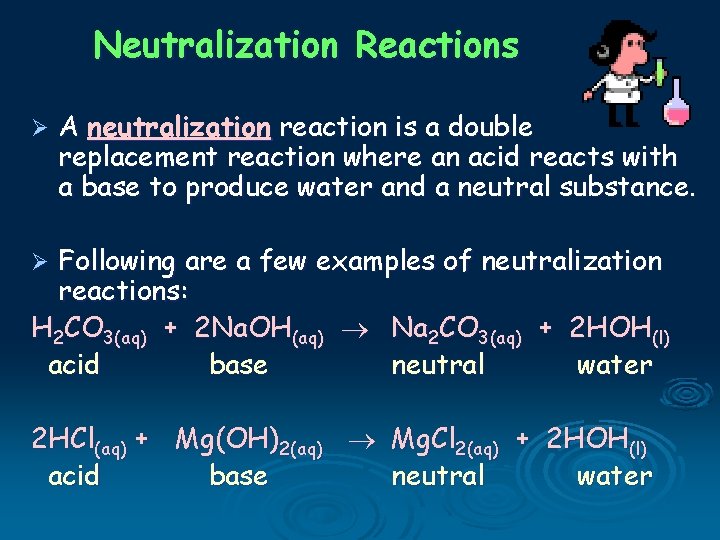
Neutralization Reactions Ø A neutralization reaction is a double replacement reaction where an acid reacts with a base to produce water and a neutral substance. Following are a few examples of neutralization reactions: H 2 CO 3(aq) + 2 Na. OH(aq) Na 2 CO 3(aq) + 2 HOH(l) acid base neutral water Ø 2 HCl(aq) + Mg(OH)2(aq) Mg. Cl 2(aq) + 2 HOH(l) acid base neutral water

Precipitates Sometimes when two soluble ionic compounds are mixed, an insoluble product is formed. Ø This insoluble product forms a solid, which is known as a precipitate. Ø The reaction to form a precipitate is a double replacement reaction. Ø