Grade 7 Science Unit 3 Mixtures Solutions The
























- Slides: 36

Grade 7 Science Unit 3: Mixtures & Solutions: The Particle Theory

What kind of coin is this? How big is it? What color is it? What is its shape? What is the texture like? What is it made of? ?

We will learn: n n n Identify different mixtures in your home and world around you Distinguish between heterogeneous and homogenous mixtures Distinguish between mixtures and pure substances using the PTM

Getting Started (page 228) http: //www. youtube. com/watch? v=h. IYdx. Quzb 60&safety_m ode=true&persist_safety_mode=1&safe=active

Mixed or Pure? n Suppose you pick up a rock on the beach. You see there are some parts of it that are grey, white, blue. You conclude that the different-coloured parts of the rock must be different types of matter. Is this a reasonable conclusion?

Mixed or Pure? n n n With a partner, select two of the following pairs of items and list as many differences as you can: Vinegar and water Aluminum foil and plastic wrap Steel and glass Molasses and cooking oil Metal paper clips and saw dust

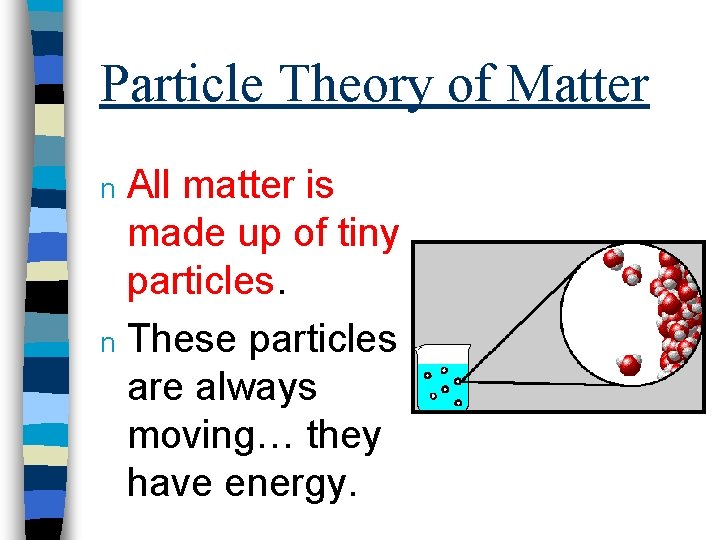
Particle Theory of Matter All matter is made up of tiny particles. n These particles are always moving… they have energy. n

n There are spaces among particles. n There attractive forces between the particles. n The particles of one substance differ from the particles of other substances.

Mixtures vs. Pure Substances Mixtures. . . n MAY have distinct visible components. n MAY appear uniform throughout.

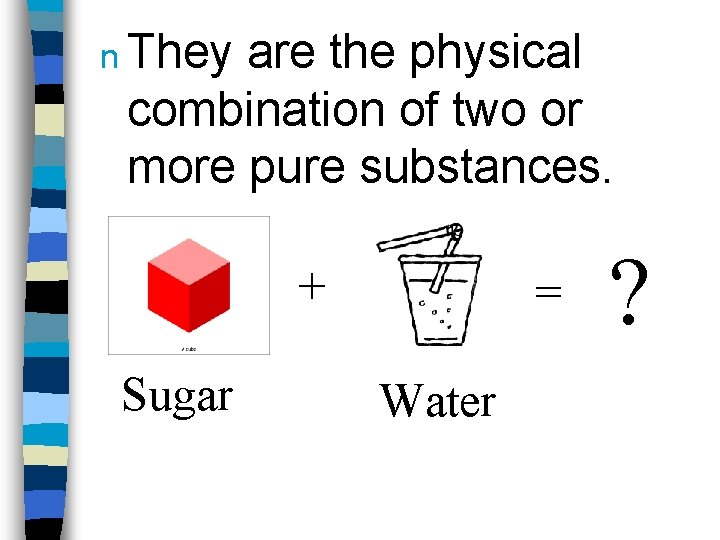
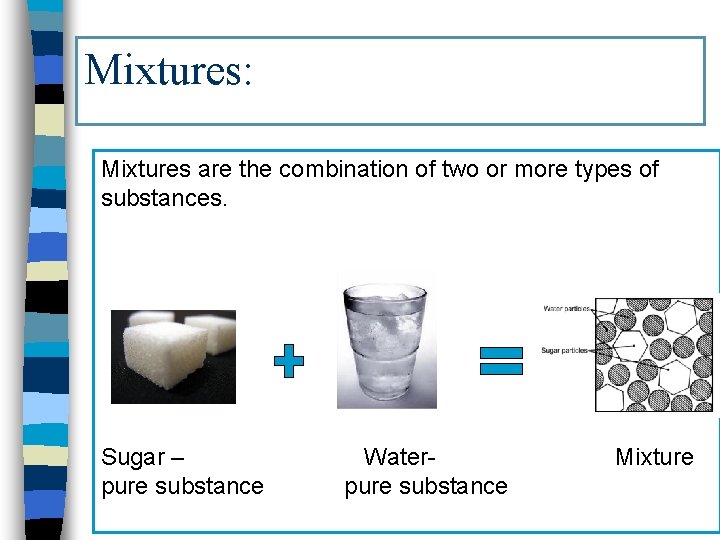
n They are the physical combination of two or more pure substances. + Sugar = Water ?

Examples of Mixtures… • salt water, kool-aid • chocolate chip cookie • muddy water • salad dressing

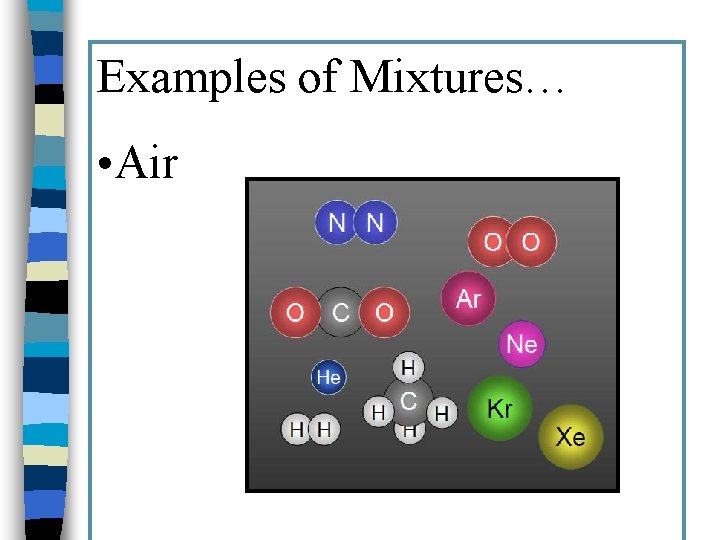
Examples of Mixtures… • Air

Pure Substances. . . n ALWAYS appear as uniform throughout n They contain either a single atom or two or more atoms chemically combined to form a different substance.

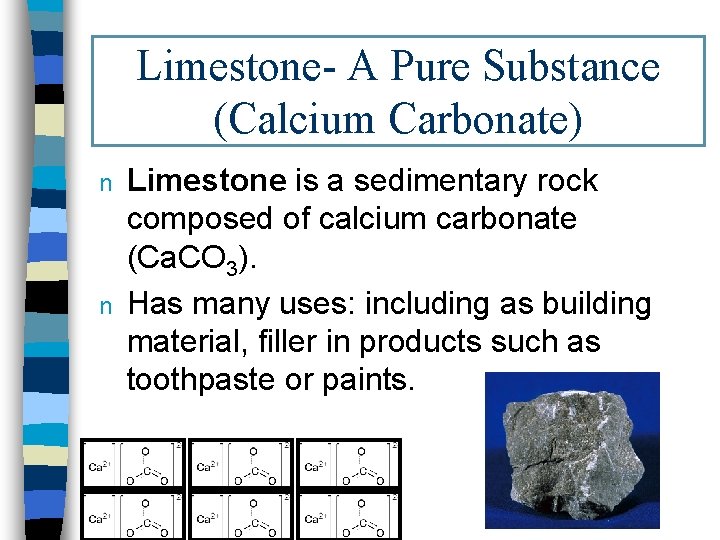
Limestone- A Pure Substance (Calcium Carbonate) n n Limestone is a sedimentary rock composed of calcium carbonate (Ca. CO 3). Has many uses: including as building material, filler in products such as toothpaste or paints.

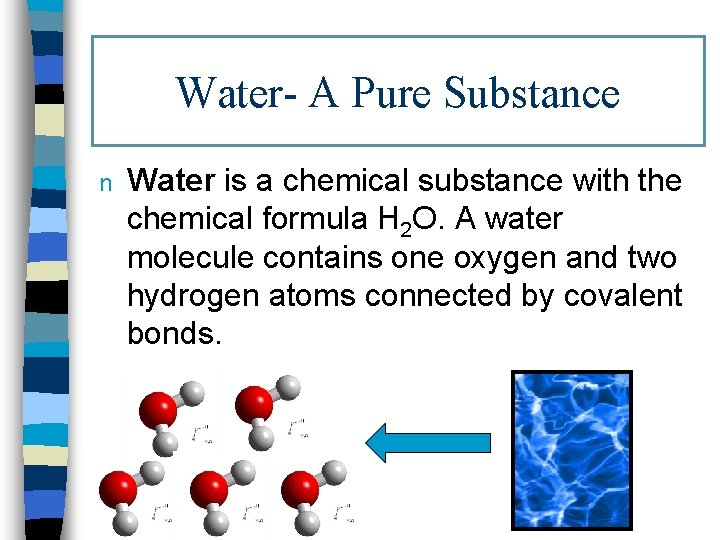
Water- A Pure Substance n Water is a chemical substance with the chemical formula H 2 O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds.

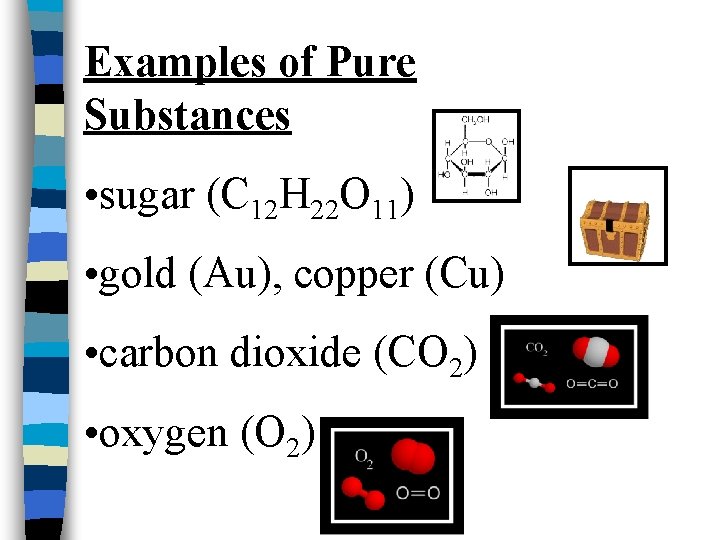
Examples of Pure Substances • sugar (C 12 H 22 O 11) • gold (Au), copper (Cu) • carbon dioxide (CO 2) • oxygen (O 2)

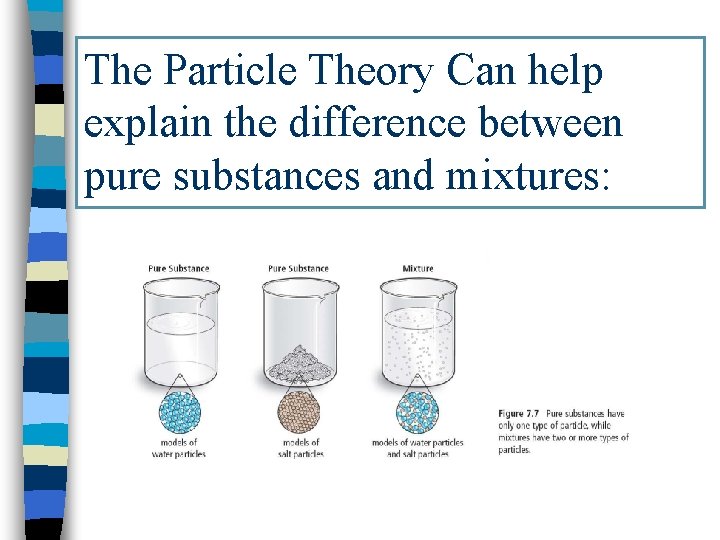
A Quick Review of chapter so far: USING THE PARTICLE THEORY OF MATTER WE CAN DISTINGUISH BETWEEN A PURE SUBSTANCE AND A MIXTURE Pure Substance: has only ONE type of particle Example: gold, iron Mixture: has 2 or more types of substance in it. We can also say it has 2 or more types of particles mixed together Example: sweetened water

Pure Substances:

Pure Substances: • Pure Substances ALWAYS appear as uniform (the same) throughout

Mixtures: Mixtures are the combination of two or more types of substances. Sugar – pure substance Waterpure substance Mixture

The Particle Theory Can help explain the difference between pure substances and mixtures:

Student Activity. . . Read pages 232, 236 and 237 Make a list of 15 -20 solutions and mixtures that you encounter in a day. * those that may pose a safety risk.

Homogeneous & Heterogeneous Mixtures

Homogeneous Mixture n also called solutions n can be solid, liquid or gas Stainless steel

n the particles are evenly mixed so that none of the original substances are visible Kool-aid

n they appear to be ONE substance n light passes through unaffected

Heterogeneous Mixtures n also Granola bar called mechanical mixtures n can be solid, liquid or gas

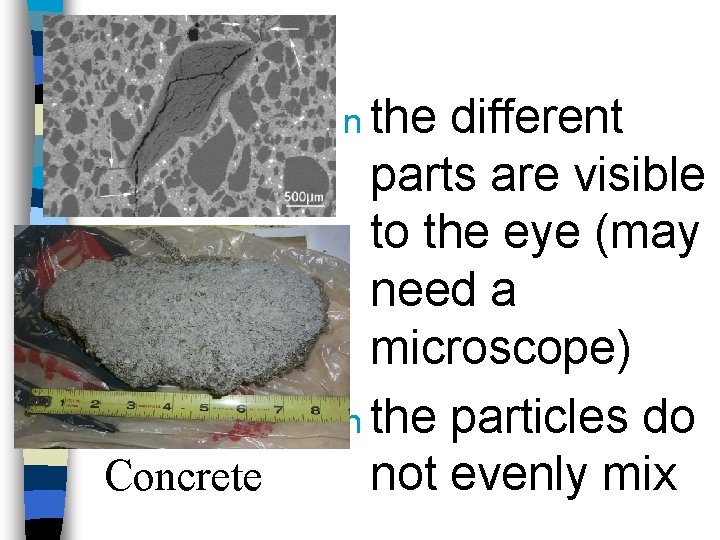
n the Concrete different parts are visible to the eye (may need a microscope) n the particles do not evenly mix

n Light will reflect perpendicular to the direction of the beam

Student Activity. . . n Create a chart and list the various homogeneous and heterogeneous mixtures in your home. n Share with your shoulder partner and add to your list.

The Tyndall Effect n. A phenomenon that can be used to distinguish between solutions and what appears to be a solution n cannot be used to distinguish between a solution and a pure liquid

In a Solution. . . n Light passes unaffected (if a student looks at the beaker perpendicular to the direction of the beam they will NOT see it

In a Mechanical Mixture. . . n The light will scatter as it passes through the mixture because all particles are not dissolved (as shown on the left)

Both Hetero and Homogenoeus Mixtures? Complete Activity 7 -1 C Page 238 & 239

Post Activity Discussion. . . Enrichment

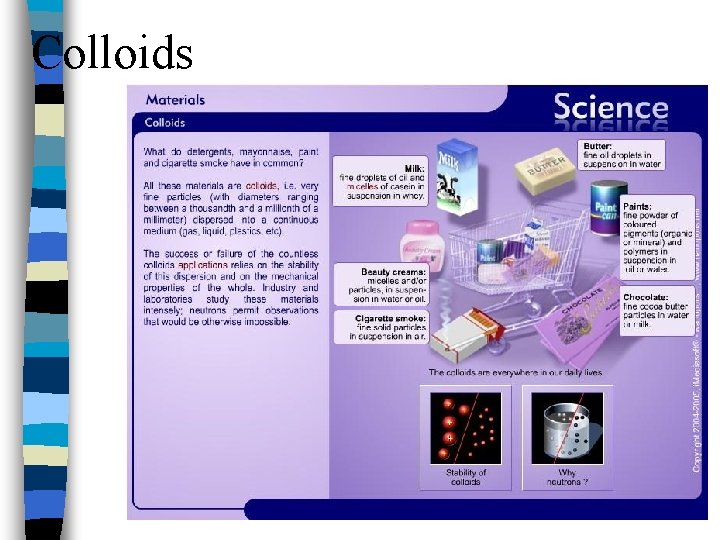
Colloids