Solution Chemistry Module 4 n A Solutions solution























- Slides: 34

Solution Chemistry Module 4

n. A Solutions solution is a homogeneous mixture of 2 or more pure substances n There are two parts to a solution n Solute: what is dissolved, smaller amount

Solubility refers to the amount of solute that will dissolve in a specific amount of solvent under certain conditions. Combination Description Example Solid dissolves in liquid Solid does not dissolve in liquid Solid is soluble Solid in insoluble Na. Cl in water Liquid dissolves in another liquid Two liquids form separate layers The liquids are Vinegar and water miscible The liquids are Oil and water immiscible Gas dissolves in liquid Gas is soluble Soda Sand in water

Factors that Affect Solubility 1. Chemical composition of the substances being mixed: “like dissolves like” 2. Temperature: solubility generally increases with temperature, opposite is true if a gas is dissolved 3. Pressure (for gases only): n Increasing pressure over solution increases gas solubility (an unopened can of soda) n Decreasing pressure over solution

“Like Dissolves Like” n Polar solutes dissolve in polar liquids n Ex: water is polar (H 2 O) and will dissolve table sugar, which is polar (C 12 H 22 O 11) n Ionic solids dissolve in strongly polar liquids n Ex: table salt (Na. Cl) will dissolve in water (H 2 O) n Nonpolar solutes dissolve in nonpolar liquids n Ex: hexane (C 6 H 14) and carbon

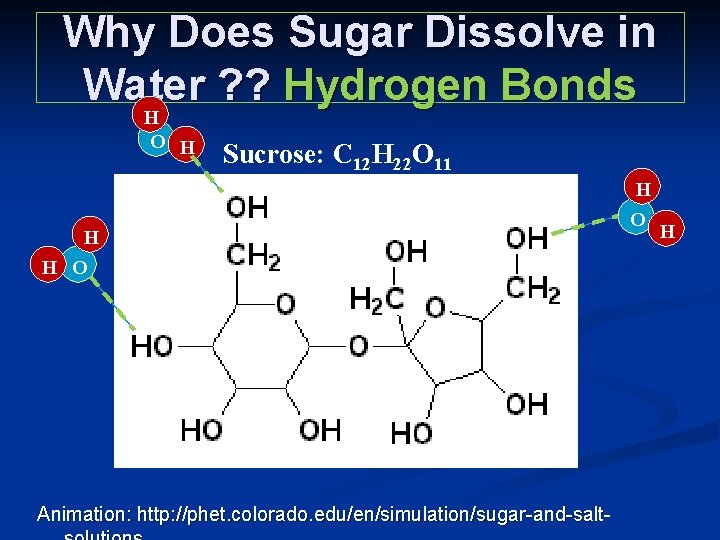
Why Does Sugar Dissolve in Water ? ? Hydrogen Bonds H O H Sucrose: C 12 H 22 O 11 H H O Animation: http: //phet. colorado. edu/en/simulation/sugar-and-salt- H O H

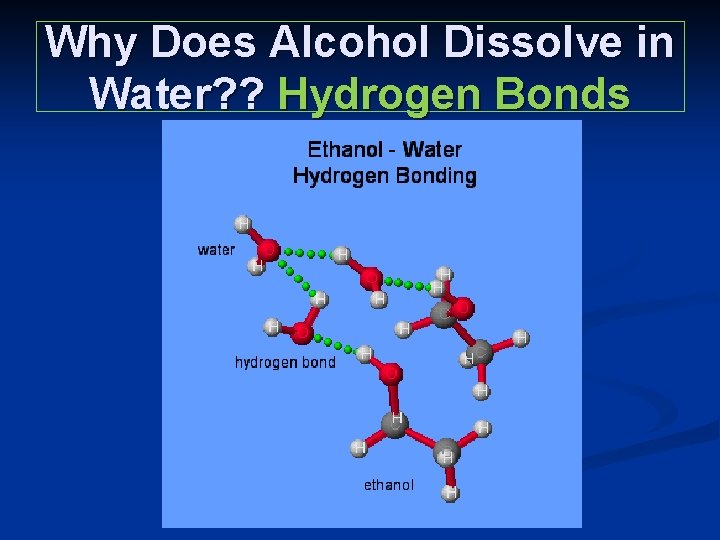
Why Does Alcohol Dissolve in Water? ? Hydrogen Bonds

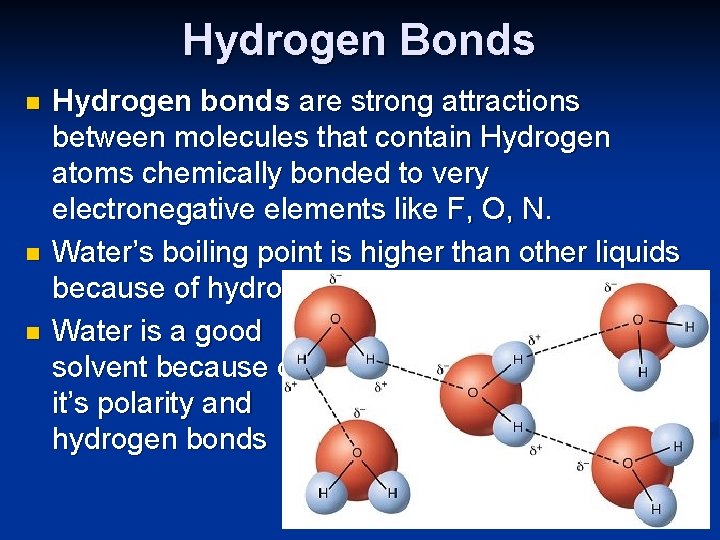
Hydrogen Bonds n n n Hydrogen bonds are strong attractions between molecules that contain Hydrogen atoms chemically bonded to very electronegative elements like F, O, N. Water’s boiling point is higher than other liquids because of hydrogen bonds Water is a good solvent because of it’s polarity and hydrogen bonds

Degrees of Saturation n Saturated solution (towel full of water) Solution has all the solute it can hold. n On a solubility curve: on the line n n Unsaturated solution (damp towel) Solution has less solute than it can hold n On solubility curve: below the line n n Supersaturated solution (dripping towel) Solution that has more solute in it than a saturated solution n Takes special conditions to do n On solubility curve: above the line n

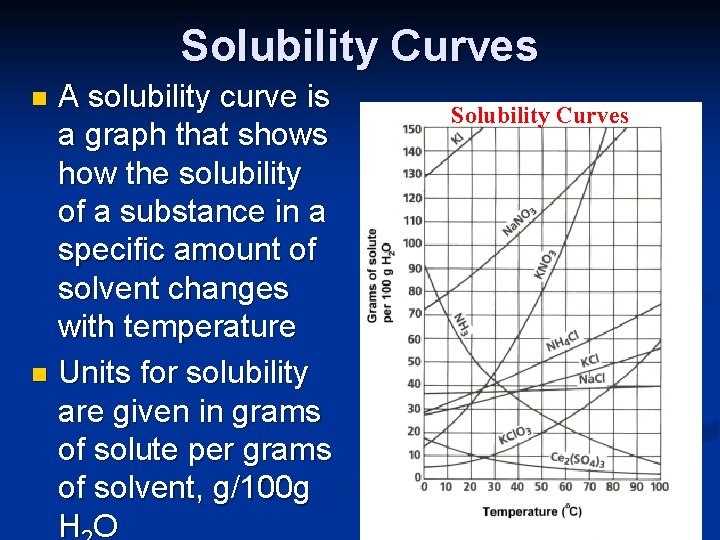
Solubility Curves A solubility curve is a graph that shows how the solubility of a substance in a specific amount of solvent changes with temperature n Units for solubility are given in grams of solute per grams of solvent, g/100 g HO n Solubility Curves

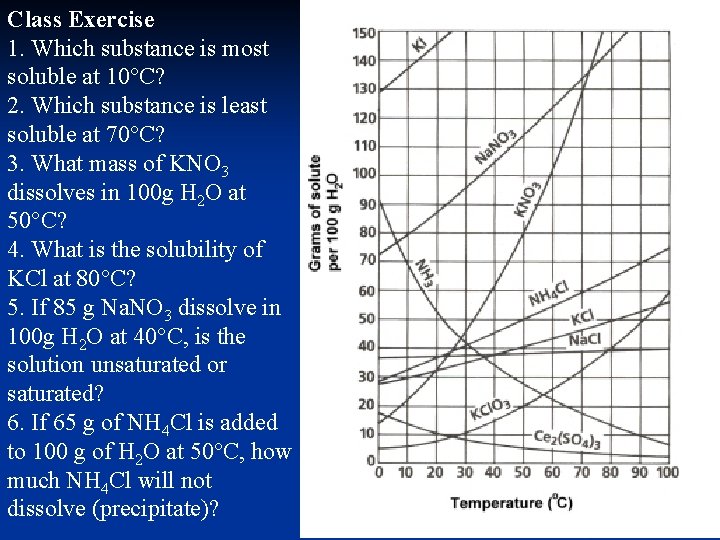
Class Exercise 1. Which substance is most soluble at 10°C? 2. Which substance is least soluble at 70°C? 3. What mass of KNO 3 dissolves in 100 g H 2 O at 50°C? 4. What is the solubility of KCl at 80°C? 5. If 85 g Na. NO 3 dissolve in 100 g H 2 O at 40°C, is the solution unsaturated or saturated? 6. If 65 g of NH 4 Cl is added to 100 g of H 2 O at 50°C, how much NH 4 Cl will not dissolve (precipitate)?

n Also Factors Affecting Rate of Solubility called rate of solution-a measure of how fast a substance dissolves 1. Solute Particle Size (surface area): smaller particle size improves solubility n 2. Stirring: speeds up solvation, particles have more contact n 3. Concentration of solution: the more solute already dissolved, the less “room” there is for more n 4. Temperature: adding heat increases particle motion and contact, increasing solubility n 5. Pressure(gases only): increasing pressure over the solution causes more gas to be dissolved n

Solution Concentration Solution concentration expresses how much solute is dissolved in a specific quantity of solution

Solution Concentration n Solutions can be dilute or concentrated n Dilute = few solute particles per amount of solvent particles n Concentrated = many solute particles per amount of solvent particles dilute concentrated : water. me. vccs. edu/courses/ENV 211/lesson 8_4 b. htm To dilute means to decrease the ratio of the number solute particles to the number of solvent particles

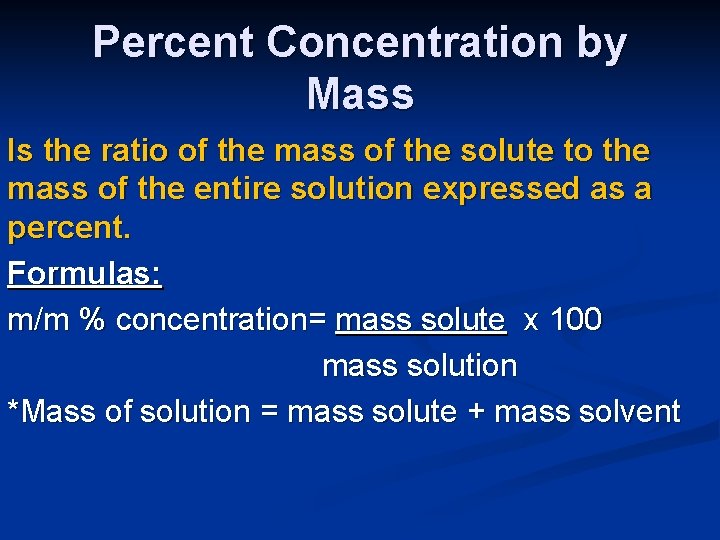
Percent Concentration by Mass Is the ratio of the mass of the solute to the mass of the entire solution expressed as a percent. Formulas: m/m % concentration= mass solute x 100 mass solution *Mass of solution = mass solute + mass solvent

Practice Problems 1. Calculate percent concentration by mass of Na. Cl for a 300 g solution that contains 4. 00 g of dissolved Na. Cl. n 2. What is the percent concentration by mass of KOH if 13. 0 g of KOH is dissolved in 260. 0 g of water? n 3. How many grams of solute are dissolved in 15 g of 5. 00 % solution? n

Answers 1. 4. 00 g Na. Cl x 100 = 1. 3 % Na. Cl 300 g soln. 2. ___13. 0 g KOH ____ x 100 = 4. 8 % Na. Cl (13. 0 g + 260. 0 g )soln. 3. . 05 = x g solute 15 g soln. Solve for x. 0. 75 g of solute.

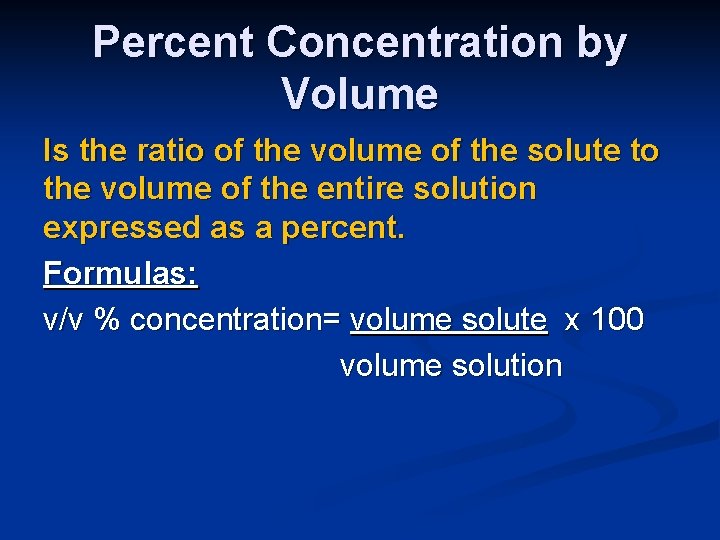
Percent Concentration by Volume Is the ratio of the volume of the solute to the volume of the entire solution expressed as a percent. Formulas: v/v % concentration= volume solute x 100 volume solution

Practice Problems n 1. Calculate percent concentration by volume of ethanol if 50 m. L of the solution contains 3. 5 m. L of ethanol. n 2. What is the percent concentration by volume of a solution made by mixing 25. 0 m. L of hydrogen peroxide with 425 m. L of water?

Answers 1. 3. 5 m. L ethanol x 100 = 7 % ethanol 50 m. L solution 2. 25. 0 m. L hydrogen peroxide x 100 = 5. 6 % (25. 0 m. L + 425 m. L ) soln.

MOLARITY (M) n n Also called molar concentration Is the number of moles of a solute dissolved in a liter of solution Formula: Molarity(M)= moles of solute L of solution

Practice Problems n 1. What is the molar concentration of a 250. 0 m. L solution containing 1. 5 moles of Na. Cl? n 2. What is the molar concentration of a 250. 0 m. L solution containing 15. 0 grams of Na. Cl? n 3. What mass of Na. Cl is dissolved in 45. 0 m. L of a 0. 75 M Na. Cl

Answers 1. 2. M = 1. 5 mol Na. Cl = 6 M Na. Cl solution 0. 2500 L solution 15 g Na. Cl x 1 mol Na. Cl = 0. 25667… mol Na. Cl 58. 44 g Na. Cl M = 0. 25667… mol Na. Cl = 1. 03 M Na. Cl solution 0. 2500 L solution 3. 0. 045 L solution x 0. 75 mol Na. Cl x 58. 44 g Na. Cl = 1. 97 g Na. Cl 1 L solution 1 mol Na. Cl

Dilutions To dilute a solution means to make the solution LESS concentrated n This means the ratio of solute particles to solvent particles decreases n n n The amount of moles of solute particles dissolved does not change Molarity initial x volume initial = molarity final x volume final n M 1 x V 1 = M 2 x V 2

Practice Problems n 1. Calculate the molarity of a solution that is formed when 750 m. L of water are added to 250 m. L of a 3. 0 M H 2 SO 4 solution. n 2. Calculate the amount of 5. 0 M HCl that would be needed to make 300. 0 m. L of 2. 0 M solution.

Answers 1. Find the final volume of the solution. V 2 = 250 m. L + 750 m. L = 1000 m. L You already have: V 1 = 250 m. L, and M 1 = 3. 0 M Substitute and solve for M 2. M 1 V 1 = M 2 V 2 (3. 0 M)(0. 25 L) = (M 2)(1 L) M 2 = 0. 75 M H 2 SO 4 2. (5. 0 M)(V 1) = (2. 0 M)(0. 3 L) V 1 = 0. 12 L or 120 m. L

Titrations A titration is a laboratory technique used to determine the concentration of a solution. n In a titration a chemist finds the amount of one solution that is required to react completely with a specific volume of a second solution. n The endpoint of a titration is reached when the number of moles of each substance are equal n

Titrations n After data are collected, the concentration of the unknown solution can be determined using the dilution equation n Macid. Vacid = Mbase. Vbase

Practice Problems 1. Calculate the concentration of hydrochloric acid solution if 23. 50 m. L are required to completely neutralize 60. 00 m. L of a potassium hydroxide (KOH) solution with a concentration of 0. 30 M. n 2. What volume of 2. 0 M sodium hydroxide solution would be required to neutralize 30. 00 m. L of 0. 50 M hydrochloric acid solution? n

Answers 1. Vacid = 23. 50 m. L Vbase = 60. 00 m. L Macid = ? ? Mbase = 0. 30 M Substitute into Ma. Va = Mb. Vb (Ma)(23. 50 m. L) = (0. 30 M)(60. 00 m. L) Ma = 0. 77 M HCl 2. Vacid = 30. 00 m. L Vbase = ? ? Macid = 0. 50 M Mbase = 2. 0 M Substitute into Ma. Va = Mb. Vb (0. 50 M)(30. 00 m. L) = (2. 0 M)(Vb)

Heterogeneous Mixtures: Suspensions n Suspension: a mixture that appears uniform while being stirred but separates into different phases when agitation stops Particles usually visible to eye (more than 1000 nm) n Can typically separate by filtration or decanting n Examples: sand in water, oil in water n

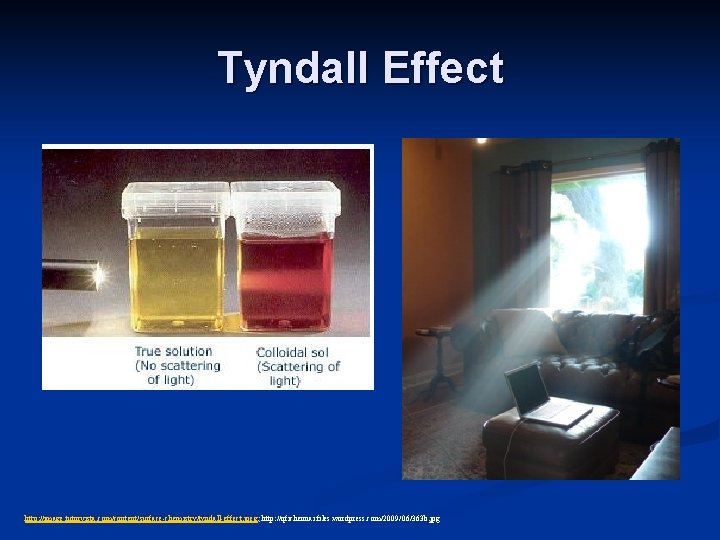
Heterogeneous Mixtures: Colloids n Colloidal dispersion: a mixture containing particles sized 1 nm to 1000 nm dispersed in a medium n Cannot separate by filtration n Not transparent (solutions are) n Exhibit the Tyndall effect -the scattering of light rays by colloidal particles n Examples: fog, smoke, soapy water, milk, blood

Tyndall Effect http: //image. tutorvista. com/content/surface-chemistry/tyndall-effect. jpeg; http: //qfichennai. files. wordpress. com/2009/06/363 b. jpg

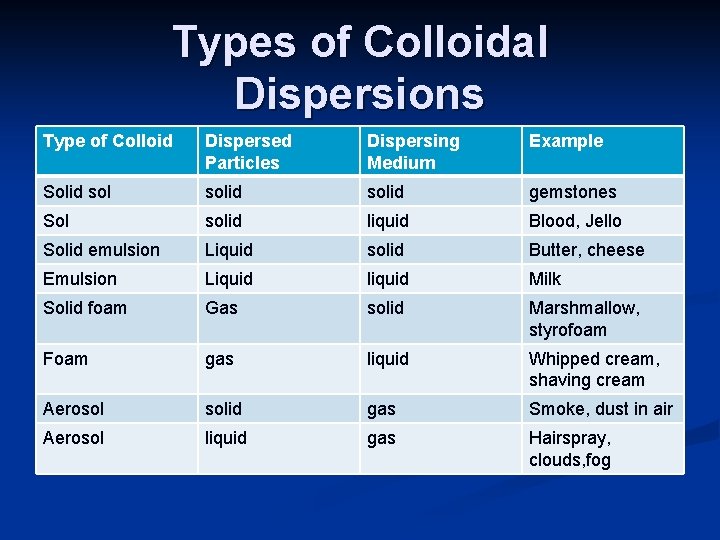
Types of Colloidal Dispersions Type of Colloid Dispersed Particles Dispersing Medium Example Solid solid gemstones Sol solid liquid Blood, Jello Solid emulsion Liquid solid Butter, cheese Emulsion Liquid liquid Milk Solid foam Gas solid Marshmallow, styrofoam Foam gas liquid Whipped cream, shaving cream Aerosol solid gas Smoke, dust in air Aerosol liquid gas Hairspray, clouds, fog