Chemistry Unit 10 Solutions Solution Definitions solution a



![Solids dissolved in liquids Gases dissolved in liquids [O Sol. 2] Sol. To As Solids dissolved in liquids Gases dissolved in liquids [O Sol. 2] Sol. To As](https://slidetodoc.com/presentation_image_h/0c6b3a2684d9bf2f3b71ab0e738dc280/image-13.jpg)






- Slides: 38

Chemistry Unit 10: Solutions

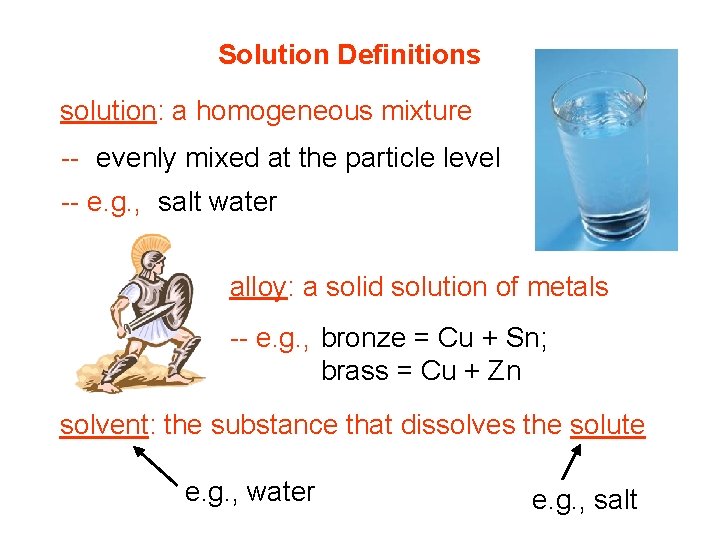
Solution Definitions solution: a homogeneous mixture -- evenly mixed at the particle level -- e. g. , salt water alloy: a solid solution of metals -- e. g. , bronze = Cu + Sn; brass = Cu + Zn solvent: the substance that dissolves the solute e. g. , water e. g. , salt

soluble: “will dissolve in” miscible: refers to two liquids that mix evenly in all proportions -- e. g. , food coloring and water

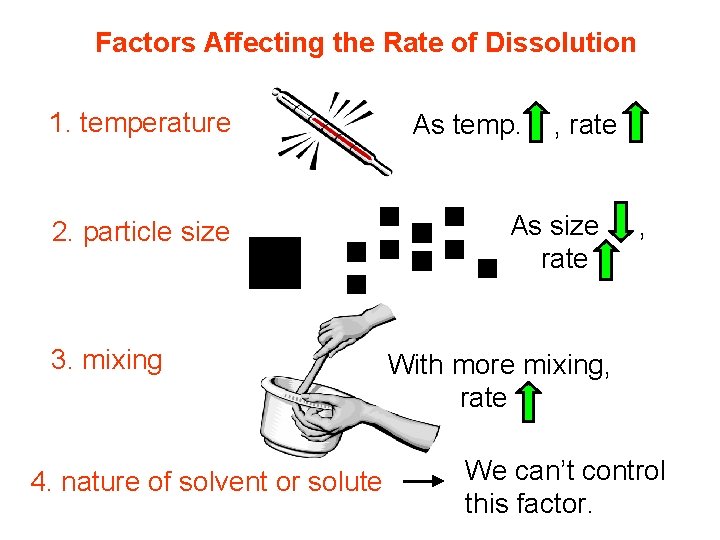
Factors Affecting the Rate of Dissolution 1. temperature 2. particle size 3. mixing 4. nature of solvent or solute As temp. , rate As size rate , With more mixing, rate We can’t control this factor.

Classes of Solutions aqueous solution: solvent = water = “the universal solvent” amalgam: solvent = mercury e. g. , dental amalgam tincture: solvent = alcohol e. g. , tincture of iodine (for cuts) organic solution: solvent carbon contains ____ Organic solvents include benzene, toluene, hexane, etc.

Non-Solution Definitions insoluble: “will NOT dissolve in” e. g. , sand water immiscible: refers to two liquids that will NOT form a solution e. g. , water and oil suspension: appears uniform while being stirred, but settles over time e. g. , liquid medications

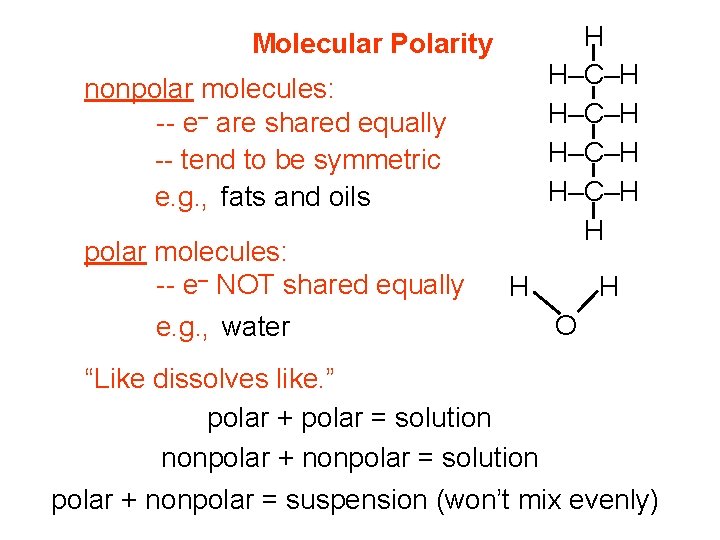
H H–C–H H Molecular Polarity nonpolar molecules: -- e– are shared equally -- tend to be symmetric e. g. , fats and oils polar molecules: -- e– NOT shared equally e. g. , water H H O “Like dissolves like. ” polar + polar = solution nonpolar + nonpolar = solution polar + nonpolar = suspension (won’t mix evenly)

Using Solubility Principles Chemicals used by body obey solubility principles. -- water-soluble vitamins: e. g. , vitamin C -- fat-soluble vitamins: e. g. , vitamins A & D Anabolic steroids and HGH are fat-soluble, synthetic hormones.

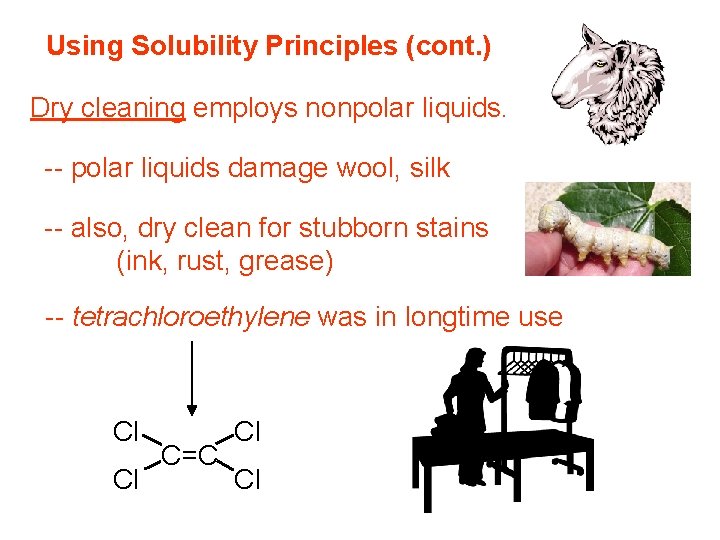
Using Solubility Principles (cont. ) Dry cleaning employs nonpolar liquids. -- polar liquids damage wool, silk -- also, dry clean for stubborn stains (ink, rust, grease) -- tetrachloroethylene was in longtime use Cl Cl C=C Cl Cl

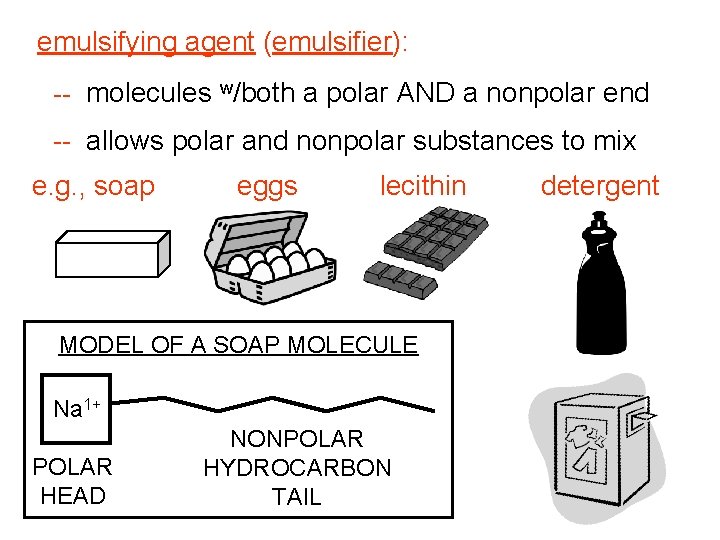
emulsifying agent (emulsifier): -- molecules w/both a polar AND a nonpolar end -- allows polar and nonpolar substances to mix e. g. , soap eggs lecithin MODEL OF A SOAP MOLECULE Na 1+ POLAR HEAD NONPOLAR HYDROCARBON TAIL detergent

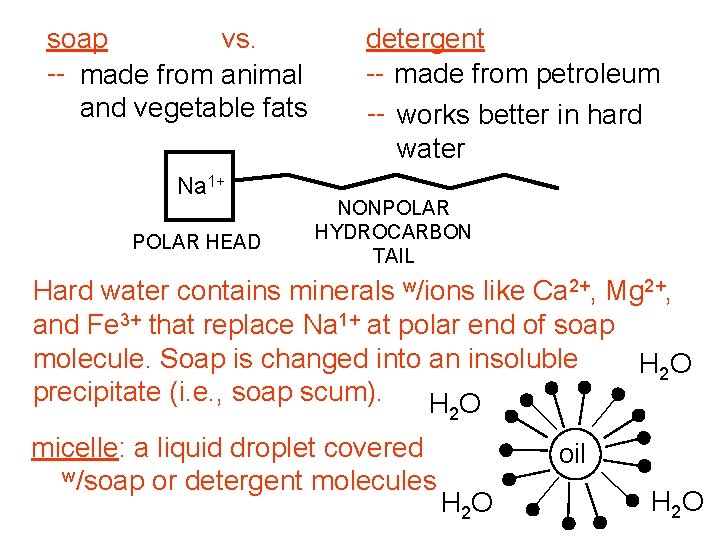
soap vs. -- made from animal and vegetable fats Na 1+ POLAR HEAD detergent -- made from petroleum -- works better in hard water NONPOLAR HYDROCARBON TAIL Hard water contains minerals w/ions like Ca 2+, Mg 2+, and Fe 3+ that replace Na 1+ at polar end of soap molecule. Soap is changed into an insoluble H 2 O precipitate (i. e. , soap scum). HO 2 micelle: a liquid droplet covered w/soap or detergent molecules oil H 2 O

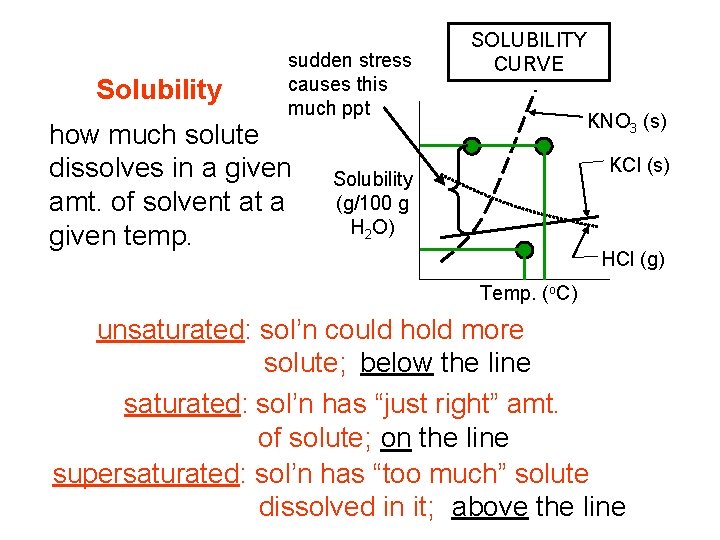
Solubility sudden stress causes this much ppt how much solute dissolves in a given amt. of solvent at a given temp. SOLUBILITY CURVE KNO 3 (s) KCl (s) Solubility (g/100 g H 2 O) HCl (g) Temp. (o. C) unsaturated: sol’n could hold more solute; below the line saturated: sol’n has “just right” amt. of solute; on the line supersaturated: sol’n has “too much” solute dissolved in it; above the line
![Solids dissolved in liquids Gases dissolved in liquids O Sol 2 Sol To As Solids dissolved in liquids Gases dissolved in liquids [O Sol. 2] Sol. To As](https://slidetodoc.com/presentation_image_h/0c6b3a2684d9bf2f3b71ab0e738dc280/image-13.jpg)
Solids dissolved in liquids Gases dissolved in liquids [O Sol. 2] Sol. To As To , solubility ___

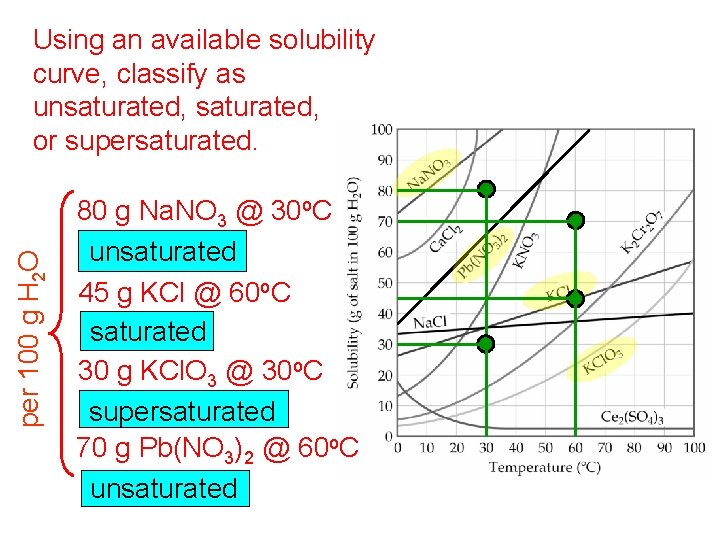
per 100 g H 2 O Using an available solubility curve, classify as unsaturated, or supersaturated. 80 g Na. NO 3 @ 30 o. C unsaturated 45 g KCl @ 60 o. C saturated 30 g KCl. O 3 @ 30 o. C supersaturated 70 g Pb(NO 3)2 @ 60 o. C unsaturated

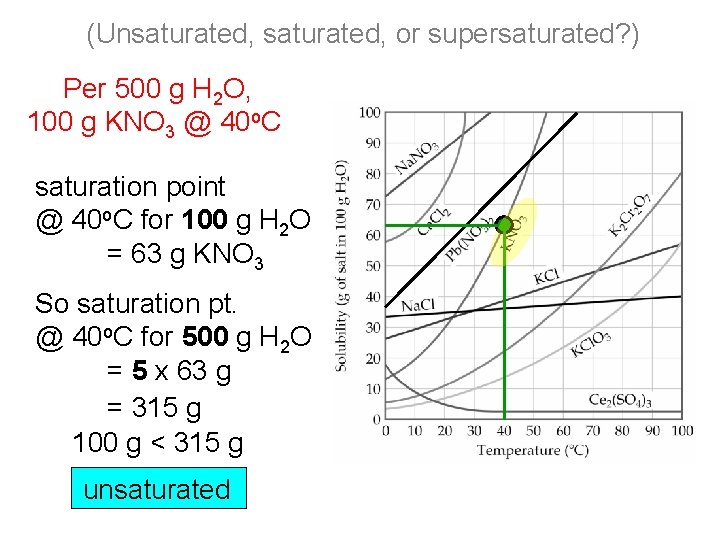
(Unsaturated, or supersaturated? ) Per 500 g H 2 O, 100 g KNO 3 @ 40 o. C saturation point @ 40 o. C for 100 g H 2 O = 63 g KNO 3 So saturation pt. @ 40 o. C for 500 g H 2 O = 5 x 63 g = 315 g 100 g < 315 g unsaturated

Describe each situation below. (A) Per 100 g H 2 O, 100 g Na. NO 3 @ 50 o. C. unsaturated; all solute dissolves; clear sol’n. (B) Cool sol’n (A) very slowly to 10 o. C. supersaturated; extra solute remains in sol’n; still clear (C) Quench sol’n (A) in an ice bath to 10 o. C. saturated; extra solute (20 g) can’t remain in sol’n and becomes visible

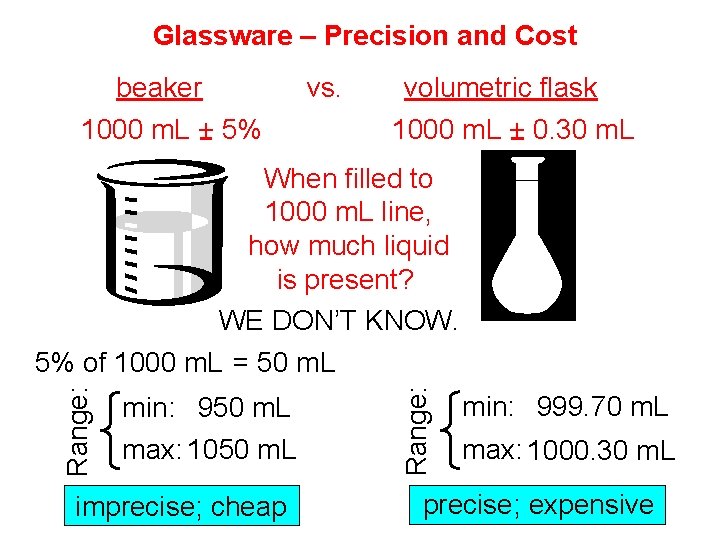
Glassware – Precision and Cost beaker vs. 1000 m. L + 5% volumetric flask 1000 m. L + 0. 30 m. L When filled to 1000 m. L line, how much liquid is present? WE DON’T KNOW. min: 950 m. L max: 1050 m. L imprecise; cheap Range: 5% of 1000 m. L = 50 m. L min: 999. 70 m. L max: 1000. 30 m. L precise; expensive

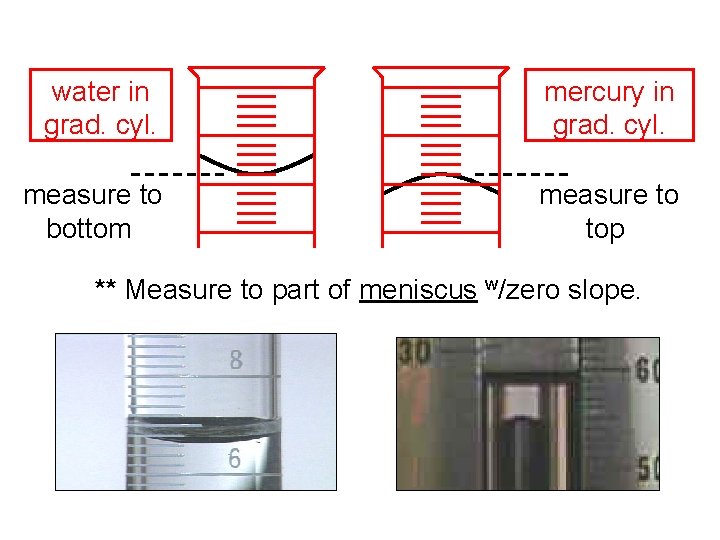
water in grad. cyl. mercury in grad. cyl. measure to bottom measure to top ** Measure to part of meniscus w/zero slope.

Concentration…a measure of solute-to-solvent ratio concentrated dilute “lots of solute” “not much solvent” “watery” Add water to dilute a sol’n; boil water off to concentrate it.

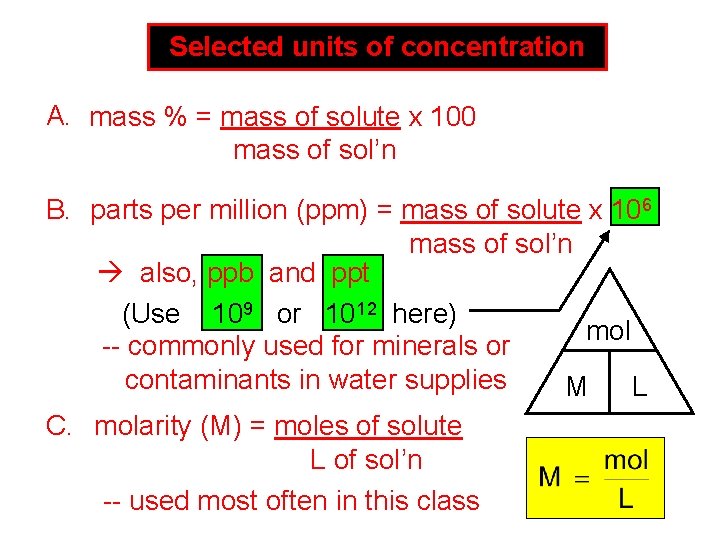
Selected units of concentration A. mass % = mass of solute x 100 mass of sol’n B. parts per million (ppm) = mass of solute x 106 mass of sol’n also, ppb and ppt (Use 109 or 1012 here) mol -- commonly used for minerals or contaminants in water supplies M L C. molarity (M) = moles of solute L of sol’n -- used most often in this class

How many mol solute are req’d to make 1. 35 L of 2. 50 M sol’n? mol = M L = 2. 50 M (1. 35 L ) = 3. 38 mol M L A. What mass sodium hydroxide is this? Na 1+ OH 1– Na. OH 3. 38 mol = 135 g Na. OH B. What mass magnesium phosphate is this? Mg 2+ PO 43– Mg 3(PO 4)2 3. 38 mol = 889 g Mg 3(PO 4)2

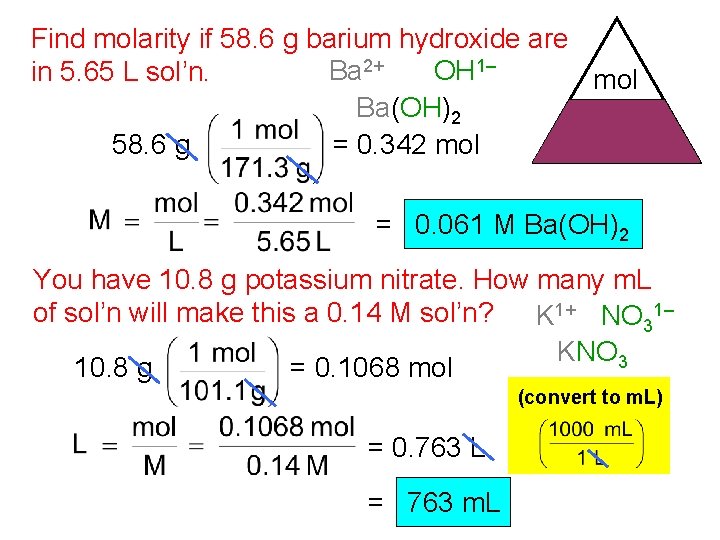
Find molarity if 58. 6 g barium hydroxide are Ba 2+ OH 1– in 5. 65 L sol’n. mol Ba(OH)2 M L 58. 6 g = 0. 342 mol = 0. 061 M Ba(OH)2 You have 10. 8 g potassium nitrate. How many m. L of sol’n will make this a 0. 14 M sol’n? K 1+ NO 31– KNO 3 10. 8 g = 0. 1068 mol (convert to m. L) = 0. 763 L = 763 m. L

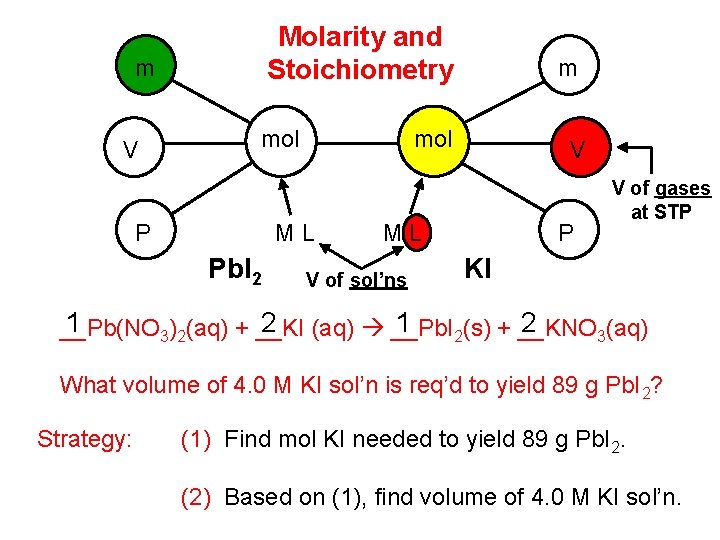
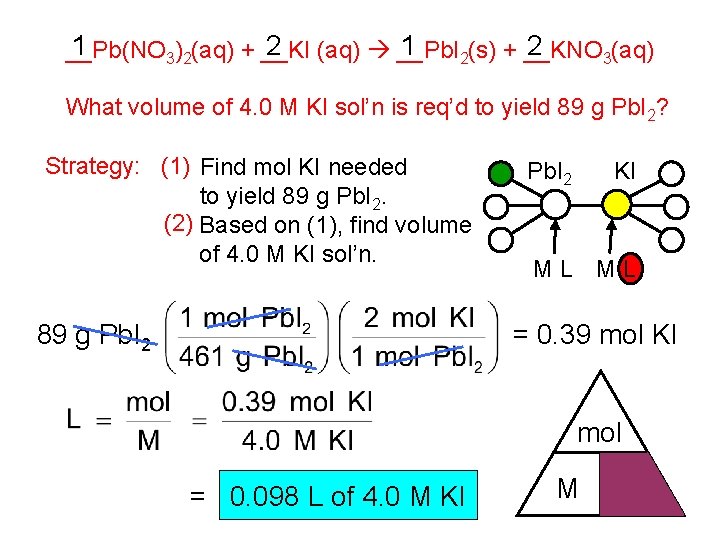
Molarity and Stoichiometry m V mol P mol ML Pb. I 2 m V ML V of sol’ns P V of gases at STP KI 1 2 __Pb(NO 3)2(aq) + __KI (aq) __Pb. I 2(s) + __KNO 3(aq) What volume of 4. 0 M KI sol’n is req’d to yield 89 g Pb. I 2? Strategy: (1) Find mol KI needed to yield 89 g Pb. I 2. (2) Based on (1), find volume of 4. 0 M KI sol’n.

1 2 __Pb(NO 3)2(aq) + __KI (aq) __Pb. I 2(s) + __KNO 3(aq) What volume of 4. 0 M KI sol’n is req’d to yield 89 g Pb. I 2? Strategy: (1) Find mol KI needed to yield 89 g Pb. I 2. (2) Based on (1), find volume of 4. 0 M KI sol’n. 89 g Pb. I 2 KI ML ML = 0. 39 mol KI mol = 0. 098 L of 4. 0 M KI M L

Cu How many m. L of a 0. 500 M Cu. SO 4 sol’n will react w/excess Al to produce 11. 0 g Cu? Cu. SO 4 Al 3+ SO 42– ML ML 3 2 3 1 __Cu. SO 4(aq) + __Al(s) __Cu(s) + __Al 2(SO 4)3(aq) 11. 0 g Cu = 0. 173 mol Cu. SO 4 = 0. 346 L = 346 m. L of 0. 500 M Cu. SO 4 mol M L

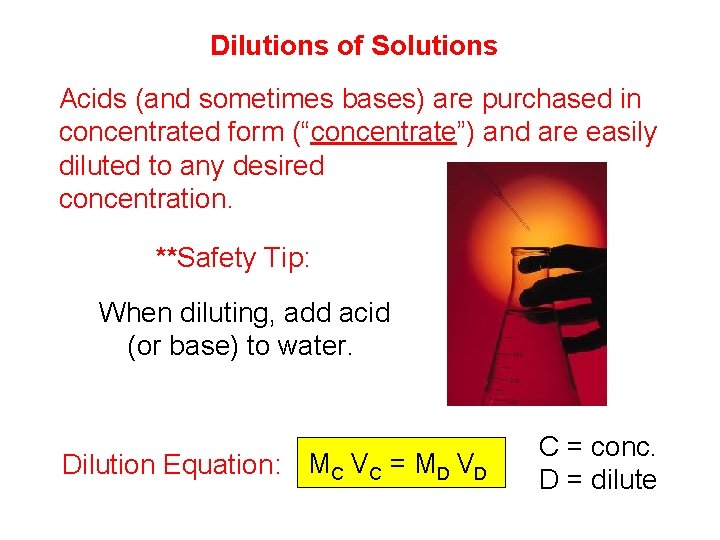
Dilutions of Solutions Acids (and sometimes bases) are purchased in concentrated form (“concentrate”) and are easily diluted to any desired concentration. **Safety Tip: When diluting, add acid (or base) to water. Dilution Equation: MC VC = MD VD C = conc. D = dilute

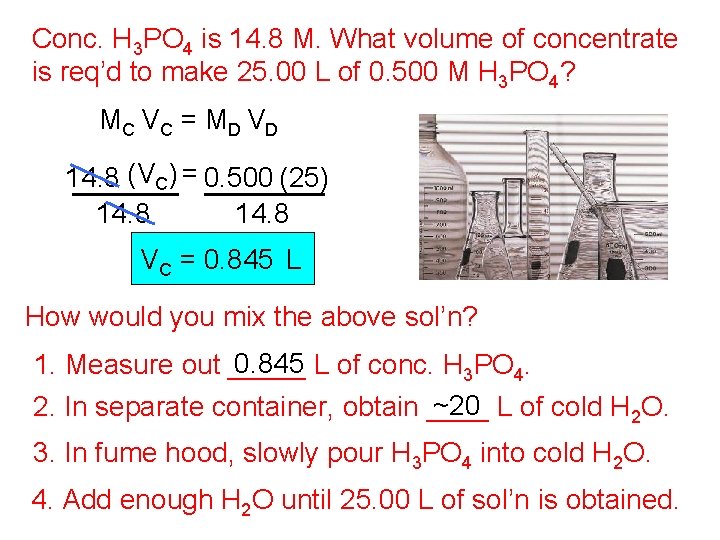
Conc. H 3 PO 4 is 14. 8 M. What volume of concentrate is req’d to make 25. 00 L of 0. 500 M H 3 PO 4? MC V C = M D V D 14. 8 (VC) = 0. 500 (25) 14. 8 VC = 0. 845 L How would you mix the above sol’n? 0. 845 L of conc. H 3 PO 4. 1. Measure out _____ ~20 L of cold H 2 O. 2. In separate container, obtain ____ 3. In fume hood, slowly pour H 3 PO 4 into cold H 2 O. 4. Add enough H 2 O until 25. 00 L of sol’n is obtained.

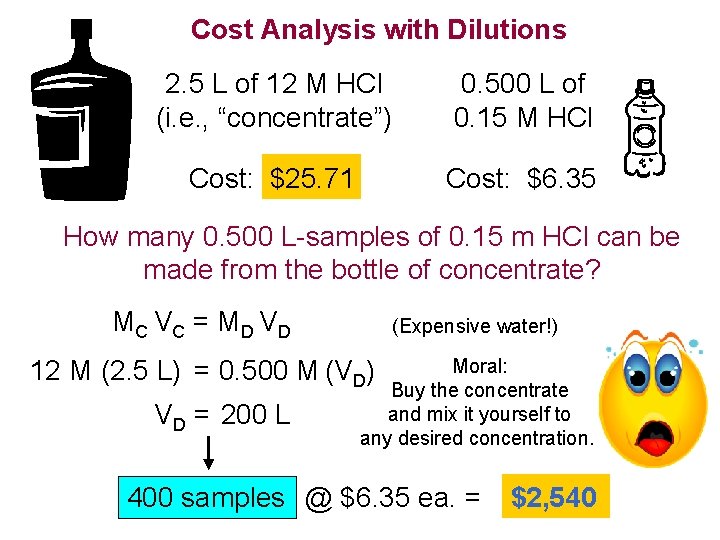
Cost Analysis with Dilutions 2. 5 L of 12 M HCl (i. e. , “concentrate”) 0. 500 L of 0. 15 M HCl Cost: $25. 71 Cost: $6. 35 How many 0. 500 L-samples of 0. 15 m HCl can be made from the bottle of concentrate? MC V C = M D V D (Expensive water!) 12 M (2. 5 L) = 0. 500 M (VD) VD = 200 L Moral: Buy the concentrate and mix it yourself to any desired concentration. 400 samples @ $6. 35 ea. = $2, 540

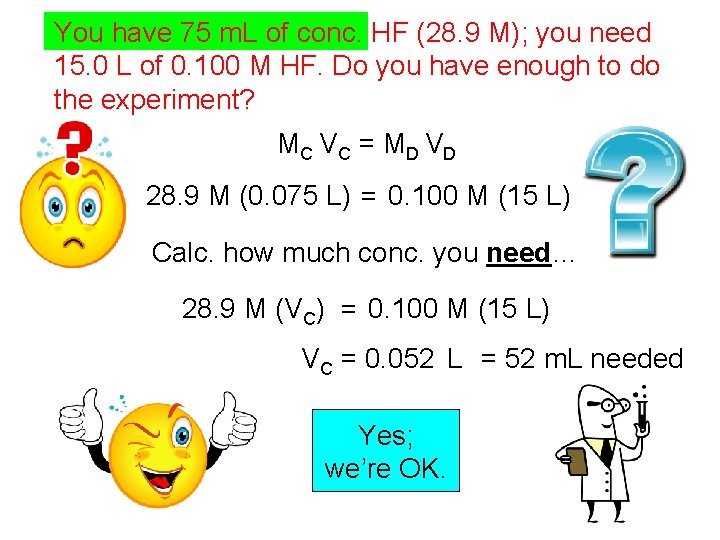
You have 75 m. L of conc. HF (28. 9 M); you need 15. 0 L of 0. 100 M HF. Do you have enough to do the experiment? MC V C = M D V D 28. 9 M (0. 075 L) = 0. 100 M (15 L) Calc. how much conc. you need… 28. 9 M (VC) = 0. 100 M (15 L) VC = 0. 052 L = 52 m. L needed Yes; we’re OK.

Dissociation occurs when neutral combinations of particles separate into ions while in aqueous solution. sodium chloride Na. Cl Na 1+ + Cl 1– sodium hydroxide Na. OH Na 1+ + OH 1– hydrochloric acid HCl H 1+ + Cl 1– sulfuric acid H 2 SO 4 2 H 1+ + SO 42– acetic acid CH 3 COOH CH 3 COO 1– + H 1+ acids yield hydrogen (H 1+) ions in aque. In general, _____ ous solution; bases _____ yield hydroxide (OH 1–) ions.

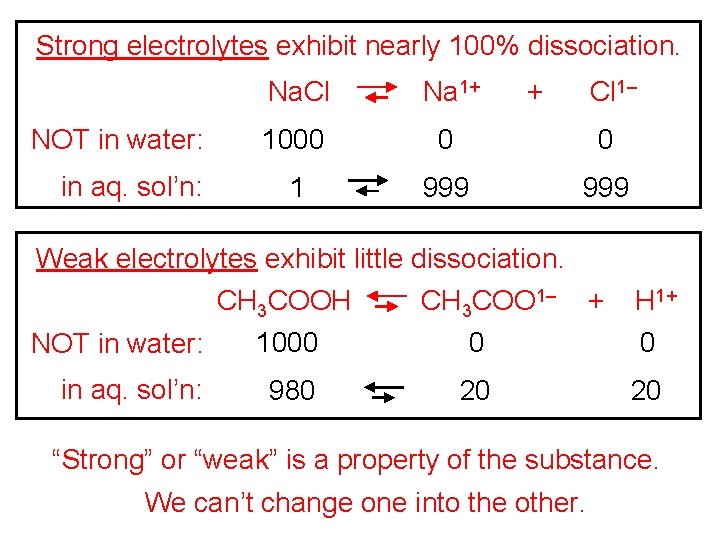
Strong electrolytes exhibit nearly 100% dissociation. NOT in water: in aq. sol’n: Na. Cl Na 1+ + Cl 1– 1000 0 0 1 999 Weak electrolytes exhibit little dissociation. CH 3 COOH CH 3 COO 1– + H 1+ NOT in water: 1000 0 0 in aq. sol’n: 980 20 20 “Strong” or “weak” is a property of the substance. We can’t change one into the other.

electrolytes: solutes that dissociate in sol’n -- conduct elec. current because of free-moving ions -- e. g. , acids, bases, most ionic compounds -- are crucial for many cellular processes -- obtained in a healthy diet -- For sustained exercise or a bout of the flu, sports drinks ensure adequate electrolytes.

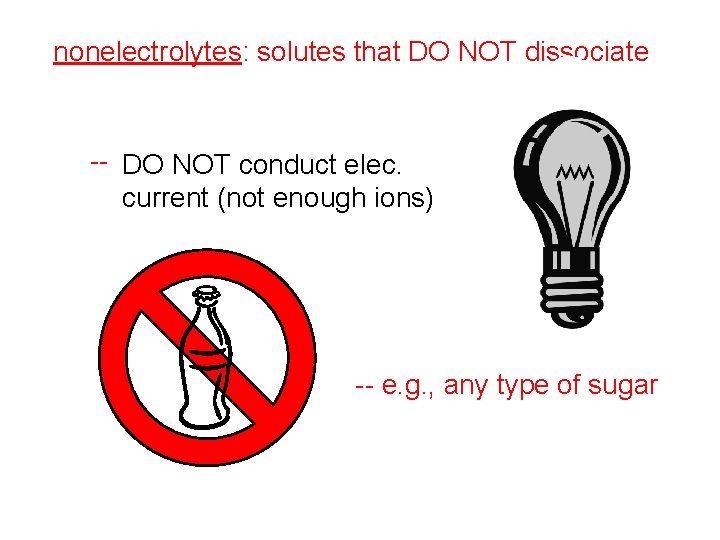
nonelectrolytes: solutes that DO NOT dissociate -- DO NOT conduct elec. current (not enough ions) -- e. g. , any type of sugar

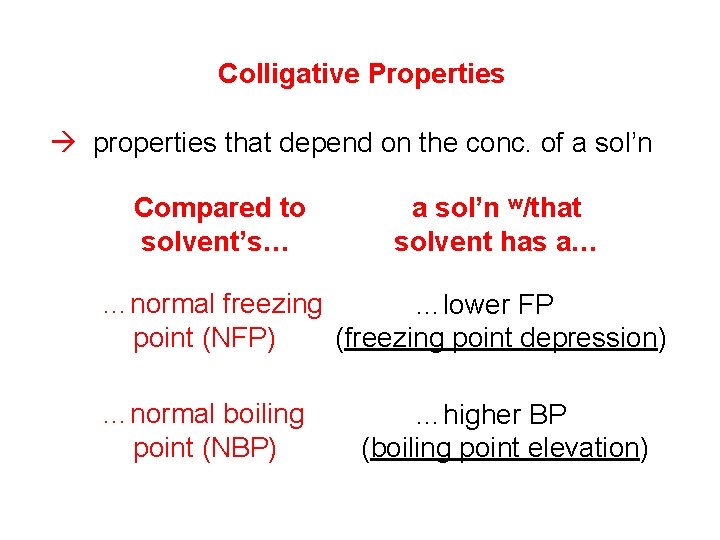
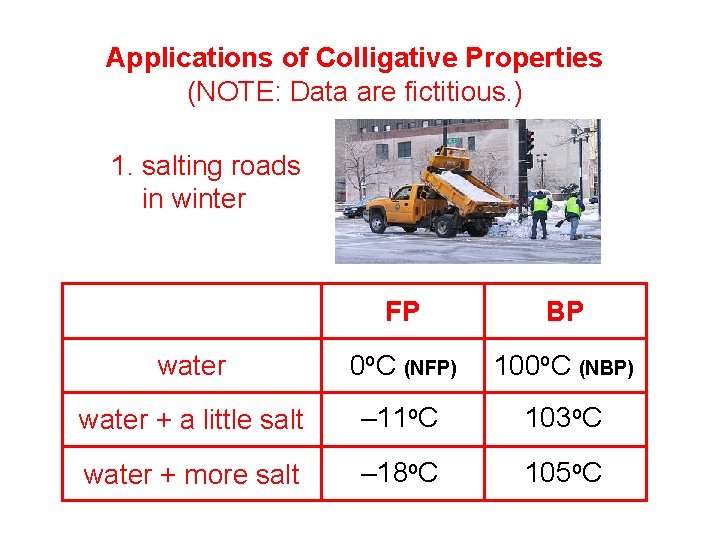
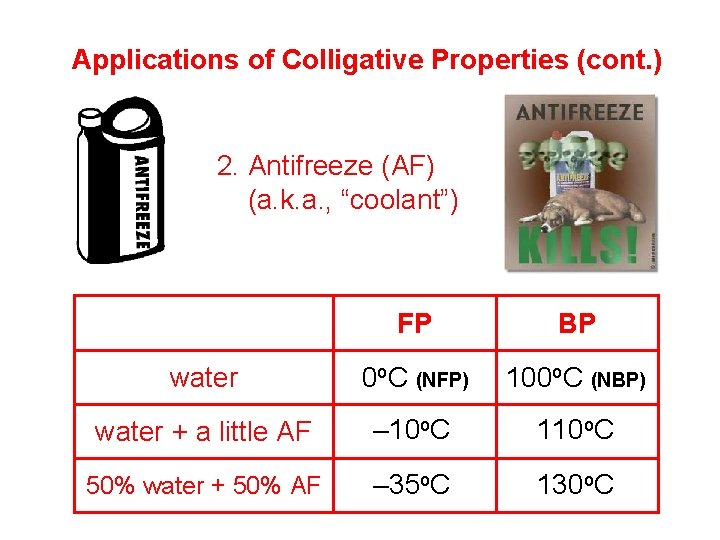
Colligative Properties properties that depend on the conc. of a sol’n Compared to solvent’s… a sol’n w/that solvent has a… …normal freezing …lower FP point (NFP) (freezing point depression) …normal boiling point (NBP) …higher BP (boiling point elevation)

Applications of Colligative Properties (NOTE: Data are fictitious. ) 1. salting roads in winter FP BP water 0 o. C (NFP) 100 o. C (NBP) water + a little salt – 11 o. C 103 o. C water + more salt – 18 o. C 105 o. C

Applications of Colligative Properties (cont. ) 2. Antifreeze (AF) (a. k. a. , “coolant”) FP BP water 0 o. C (NFP) 100 o. C (NBP) water + a little AF – 10 o. C 110 o. C 50% water + 50% AF – 35 o. C 130 o. C

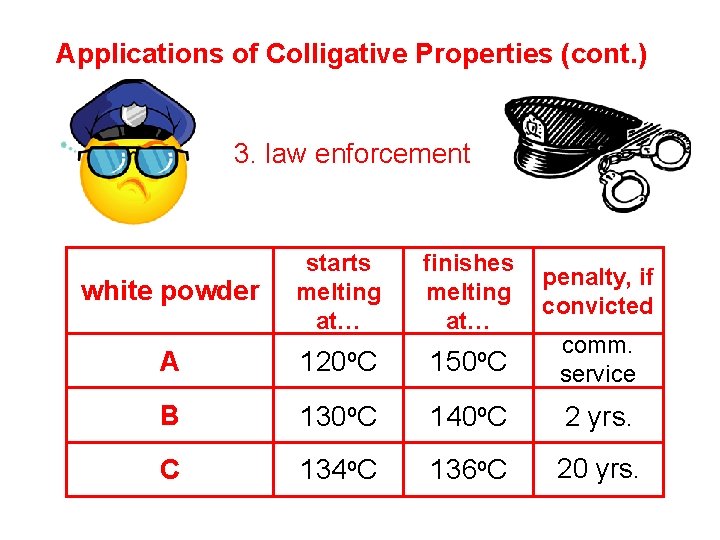
Applications of Colligative Properties (cont. ) 3. law enforcement white powder starts melting at… finishes melting at… A 120 o. C 150 o. C comm. service B 130 o. C 140 o. C 2 yrs. C 134 o. C 136 o. C 20 yrs. penalty, if convicted

h h