Calculating Rate of Reaction Calculating Rate Recall rate




- Slides: 16

Calculating Rate of Reaction

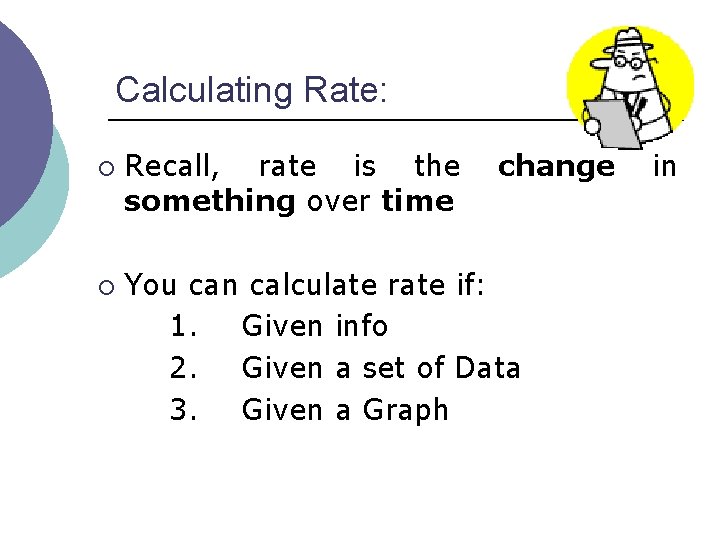
Calculating Rate: ¡ ¡ Recall, rate is the something over time change You can calculate rate if: 1. Given info 2. Given a set of Data 3. Given a Graph in


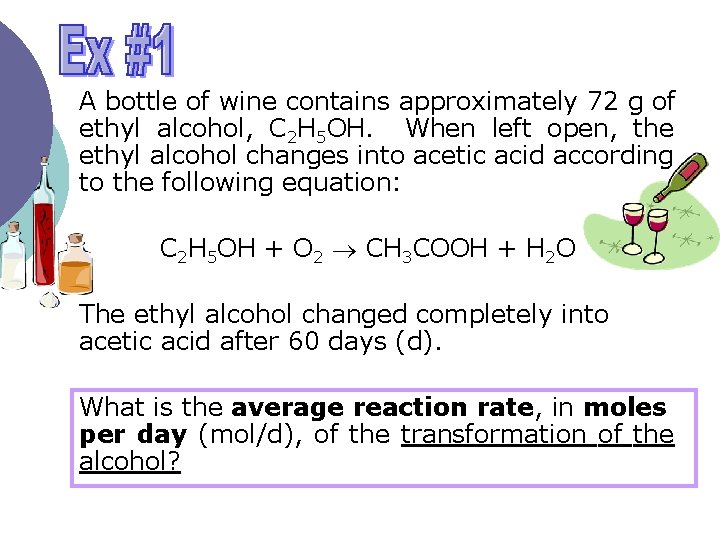
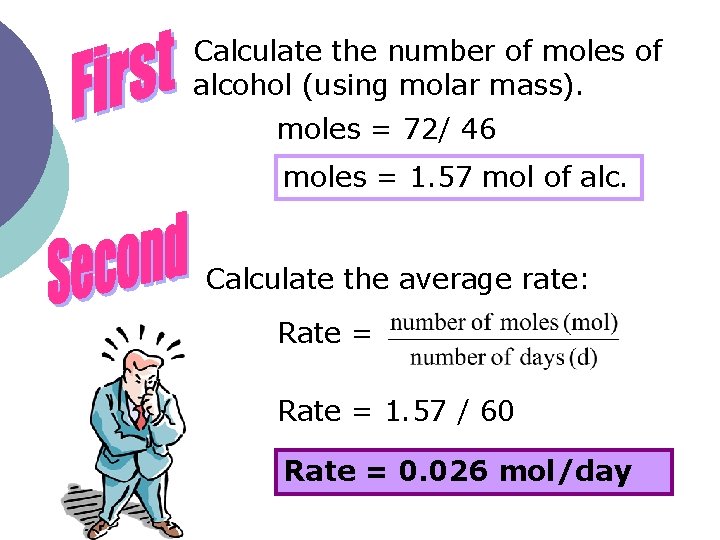
A bottle of wine contains approximately 72 g of ethyl alcohol, C 2 H 5 OH. When left open, the ethyl alcohol changes into acetic acid according to the following equation: C 2 H 5 OH + O 2 CH 3 COOH + H 2 O The ethyl alcohol changed completely into acetic acid after 60 days (d). What is the average reaction rate, in moles per day (mol/d), of the transformation of the alcohol?

Calculate the number of moles of alcohol (using molar mass). moles = 72/ 46 moles = 1. 57 mol of alc. Calculate the average rate: Rate = 1. 57 / 60 Rate = 0. 026 mol/day

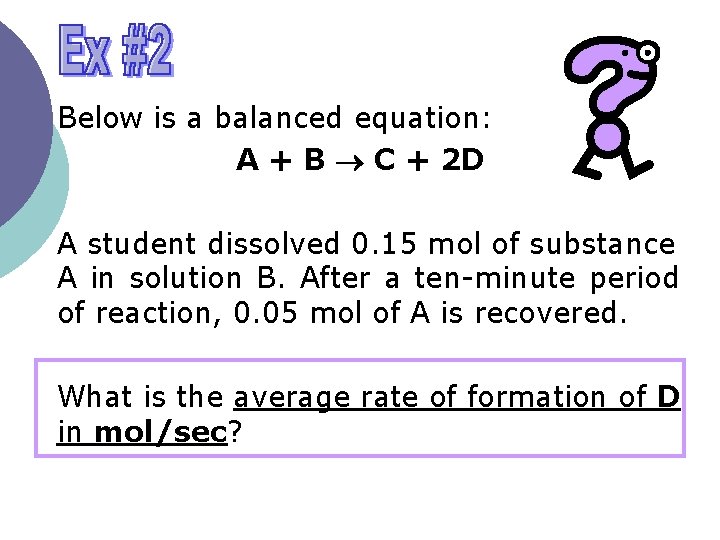
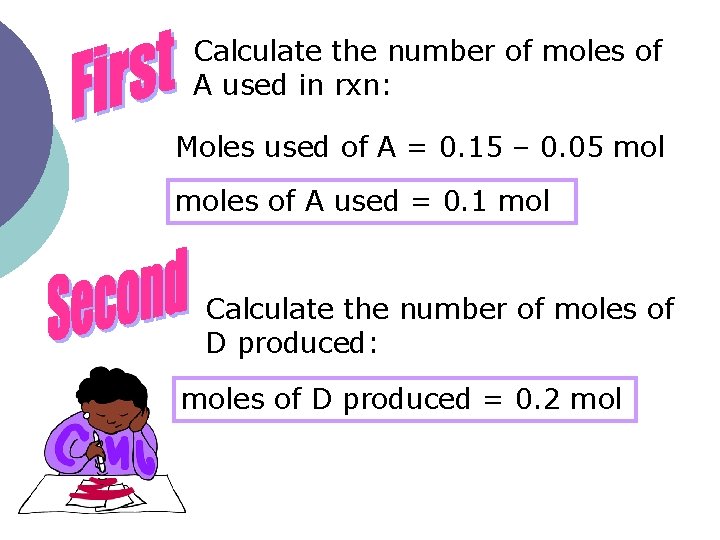
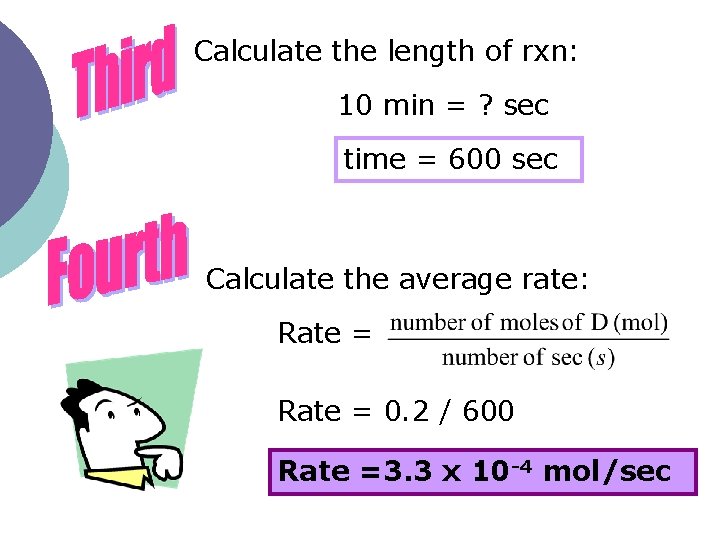
Below is a balanced equation: A + B C + 2 D A student dissolved 0. 15 mol of substance A in solution B. After a ten-minute period of reaction, 0. 05 mol of A is recovered. What is the average rate of formation of D in mol/sec?

Calculate the number of moles of A used in rxn: Moles used of A = 0. 15 – 0. 05 moles of A used = 0. 1 mol Calculate the number of moles of D produced: moles of D produced = 0. 2 mol

Calculate the length of rxn: 10 min = ? sec time = 600 sec Calculate the average rate: Rate = 0. 2 / 600 Rate =3. 3 x 10 -4 mol/sec


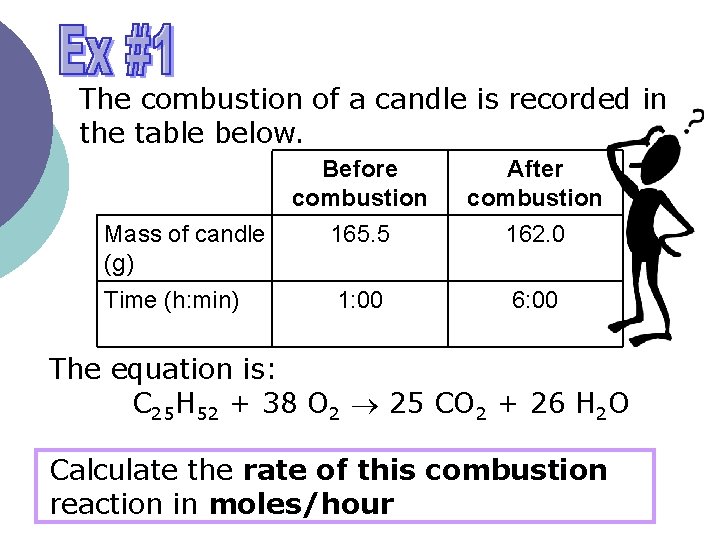
The combustion of a candle is recorded in the table below. Mass of candle (g) Time (h: min) Before combustion 165. 5 After combustion 162. 0 1: 00 6: 00 The equation is: C 25 H 52 + 38 O 2 25 CO 2 + 26 H 2 O Calculate the rate of this combustion reaction in moles/hour

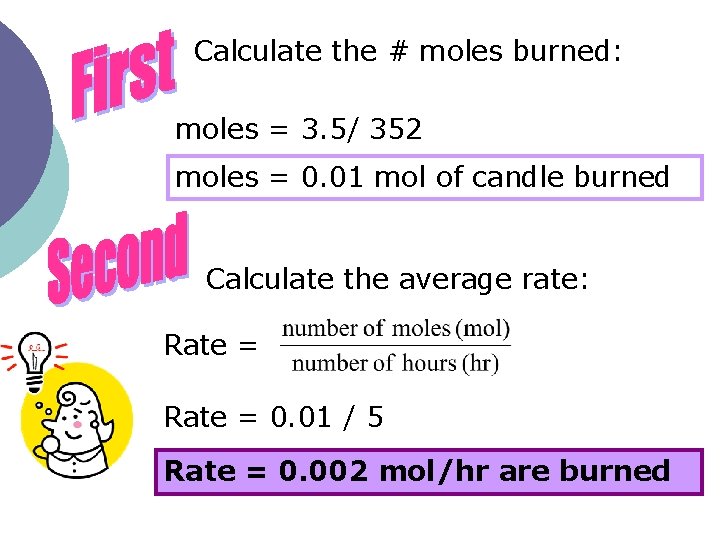
Calculate the # moles burned: moles = 3. 5/ 352 moles = 0. 01 mol of candle burned Calculate the average rate: Rate = 0. 01 / 5 Rate = 0. 002 mol/hr are burned


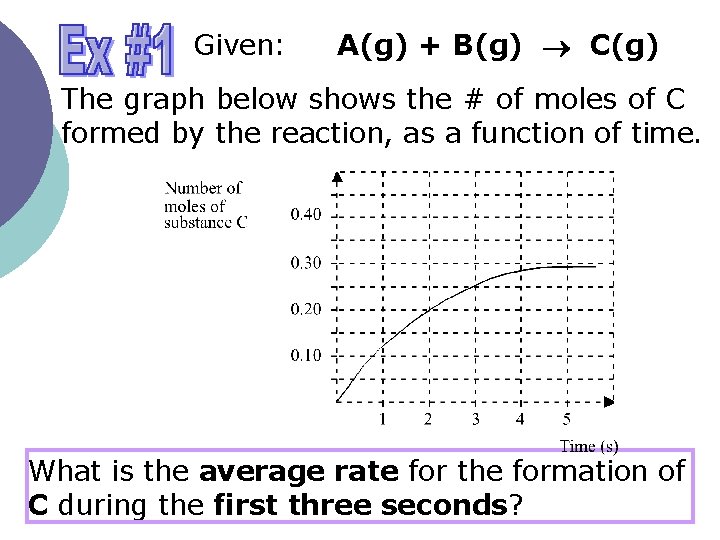
Given: A(g) + B(g) C(g) The graph below shows the # of moles of C formed by the reaction, as a function of time. What is the average rate for the formation of C during the first three seconds?

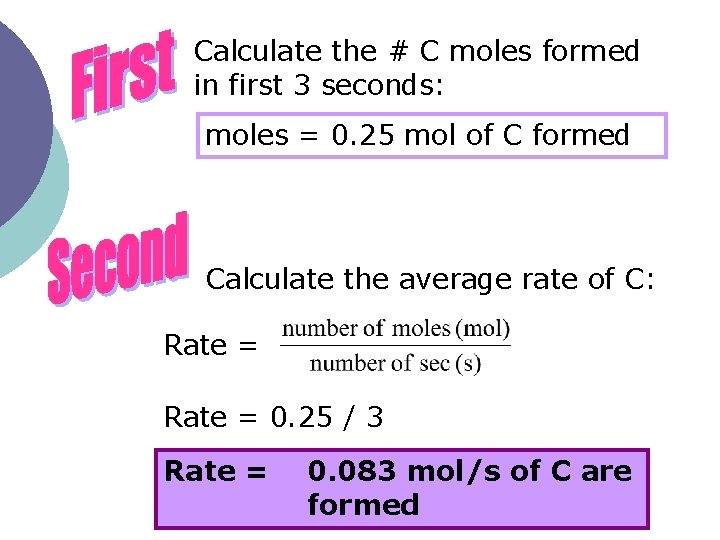
Calculate the # C moles formed in first 3 seconds: moles = 0. 25 mol of C formed Calculate the average rate of C: Rate = 0. 25 / 3 Rate = 0. 083 mol/s of C are formed

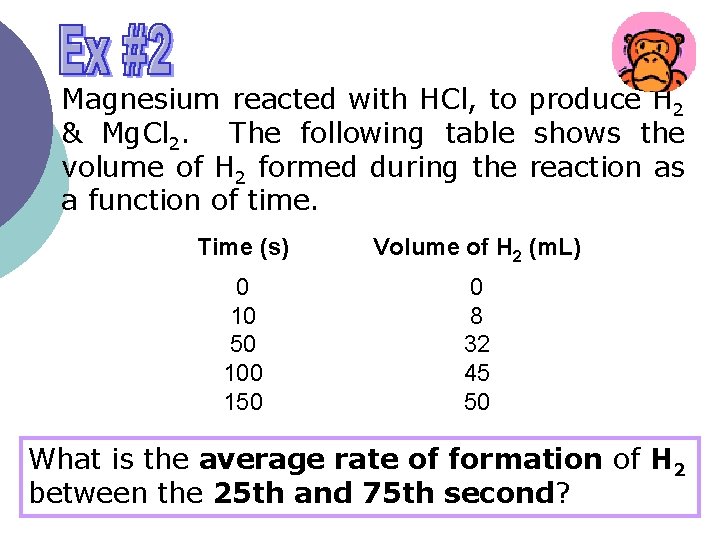
Magnesium reacted with HCl, to produce H 2 & Mg. Cl 2. The following table shows the volume of H 2 formed during the reaction as a function of time. Time (s) Volume of H 2 (m. L) 0 10 50 100 150 0 8 32 45 50 What is the average rate of formation of H 2 between the 25 th and 75 th second?

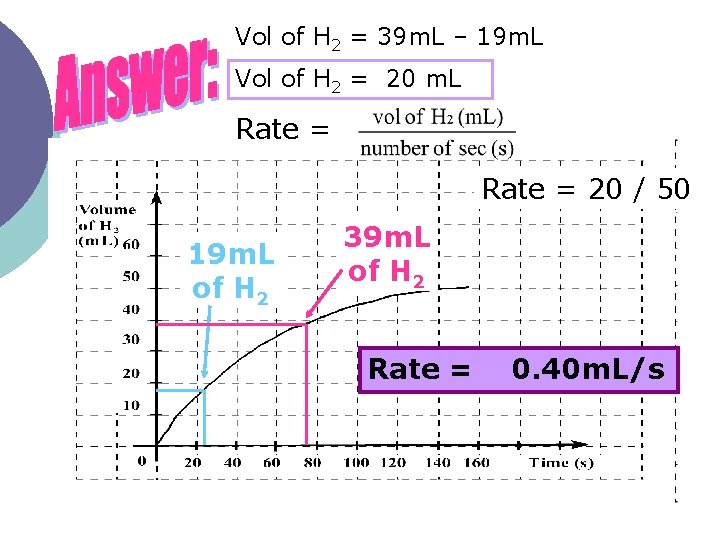
Vol of H 2 = 39 m. L – 19 m. L Vol of H 2 = 20 m. L Rate = 20 / 50 19 m. L of H 2 39 m. L of H 2 Rate = 0. 40 m. L/s
 Reaction rate equation
Reaction rate equation Determine enthalpy of reaction
Determine enthalpy of reaction 17.4 calculating heats of reaction
17.4 calculating heats of reaction Overapplied overhead
Overapplied overhead Drop factor calculation
Drop factor calculation Calculating infusion time and completion time calculator
Calculating infusion time and completion time calculator What is meant by machine hour rate
What is meant by machine hour rate Drop rate formula
Drop rate formula Gtt/min formula
Gtt/min formula Calculating average rate of change
Calculating average rate of change Calculating rate of infusion
Calculating rate of infusion Iv flow rate
Iv flow rate Calculate inflation rate
Calculate inflation rate How to calculate absorption cost per unit
How to calculate absorption cost per unit Difference between nuclear reaction and chemical reaction
Difference between nuclear reaction and chemical reaction Leukoerythroblastic reaction vs leukemoid reaction
Leukoerythroblastic reaction vs leukemoid reaction E1cb elimination reaction
E1cb elimination reaction