Collision Theory and Reaction Rates Collision Theory Chemical








- Slides: 19

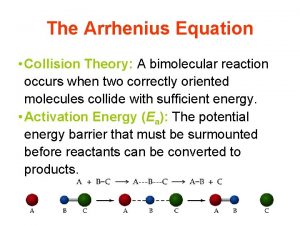
Collision Theory and Reaction Rates

Collision Theory • Chemical reactions only occur when atoms, ions, or molecules collide. • The species must make contact for a reaction to occur. • Collisions must have proper orientation and sufficient energy to be successful.

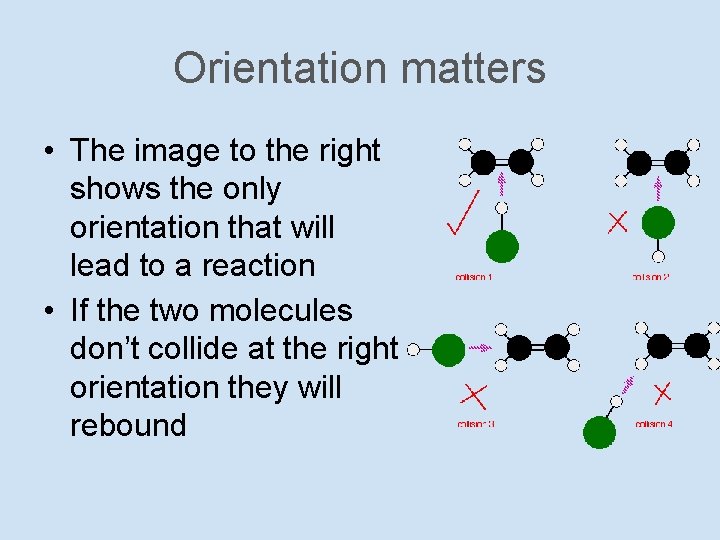
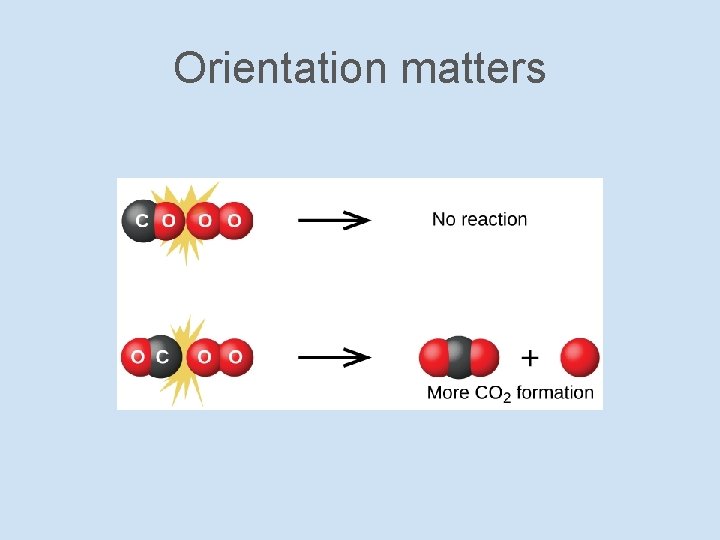
Orientation matters • The image to the right shows the only orientation that will lead to a reaction • If the two molecules don’t collide at the right orientation they will rebound

Orientation matters

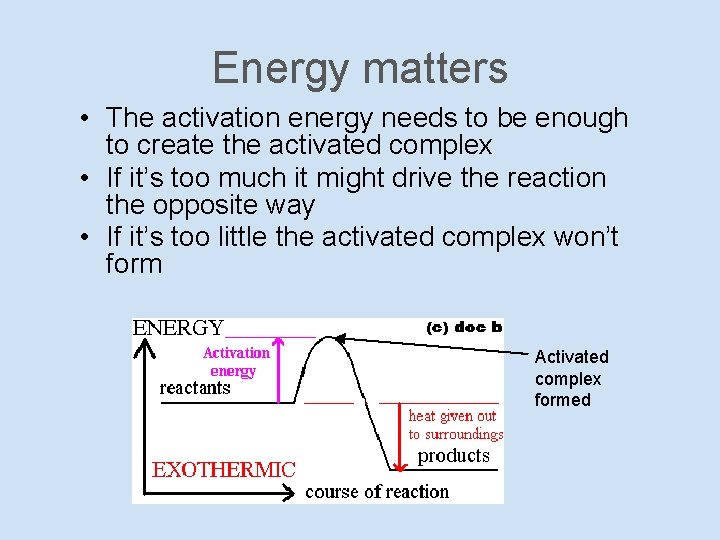
Energy matters • The activation energy needs to be enough to create the activated complex • If it’s too much it might drive the reaction the opposite way • If it’s too little the activated complex won’t form Activated complex formed

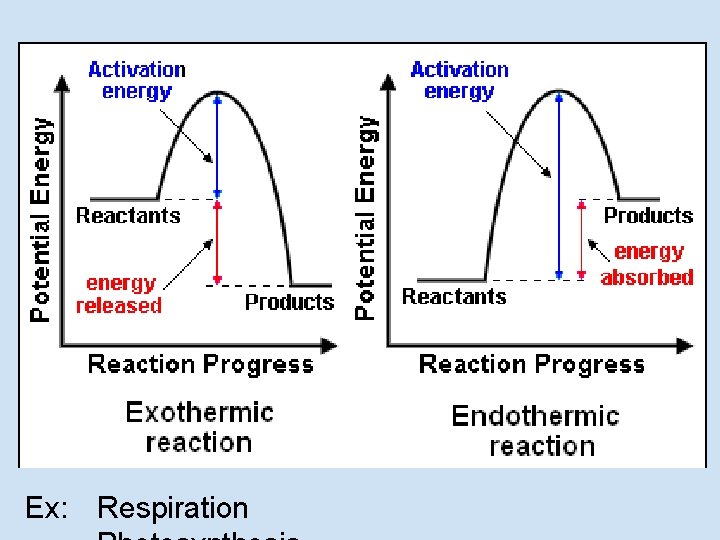
Ex: Respiration

Other connections • The graph you saw for the activation energy helps you to identify if a reaction is exothermic or endothermic • The activation energy is connected to the overall energy of the reaction • It takes energy to break bonds (Activation energy) and when bonds are reformed some energy is released.

Quiz check! True or false: • As long as there is enough energy the reaction will go forward • A poor orientation can be overcome with more energy • When a product is being formed and is in a high energy intermediate step we call that a. b. c. d. Homogenous reaction Heterogeneous reaction Activated complex Rebounded actuality

Reaction Rates ➢ The rate of a chemical reaction is affected by many factors, including: ● Concentration ● Pressure ● Temperature ● Surface area ● Catalyst

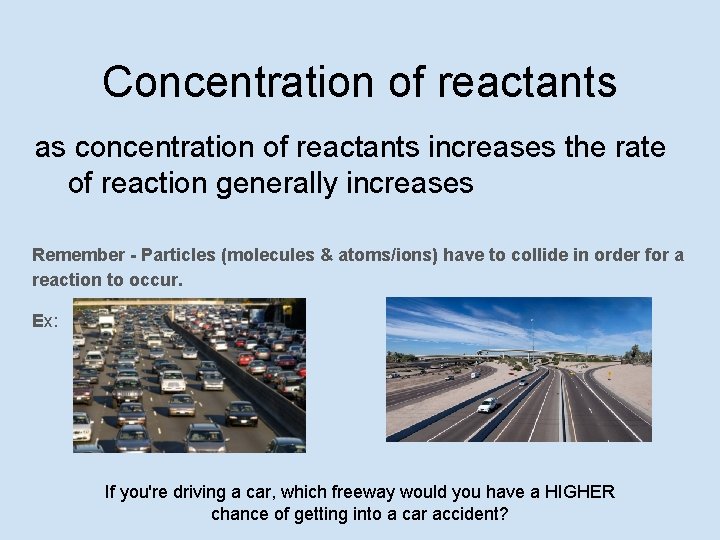
Concentration of reactants as concentration of reactants increases the rate of reaction generally increases Remember - Particles (molecules & atoms/ions) have to collide in order for a reaction to occur. Ex: If you're driving a car, which freeway would you have a HIGHER chance of getting into a car accident?

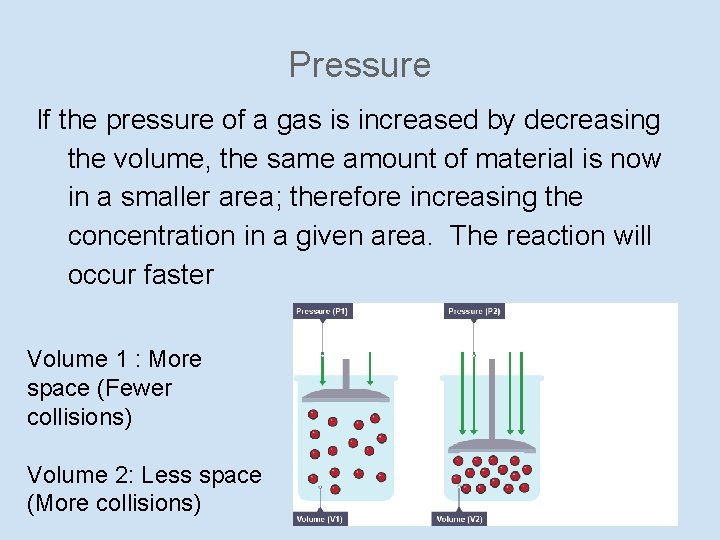
Pressure If the pressure of a gas is increased by decreasing the volume, the same amount of material is now in a smaller area; therefore increasing the concentration in a given area. The reaction will occur faster Volume 1 : More space (Fewer collisions) Volume 2: Less space (More collisions)

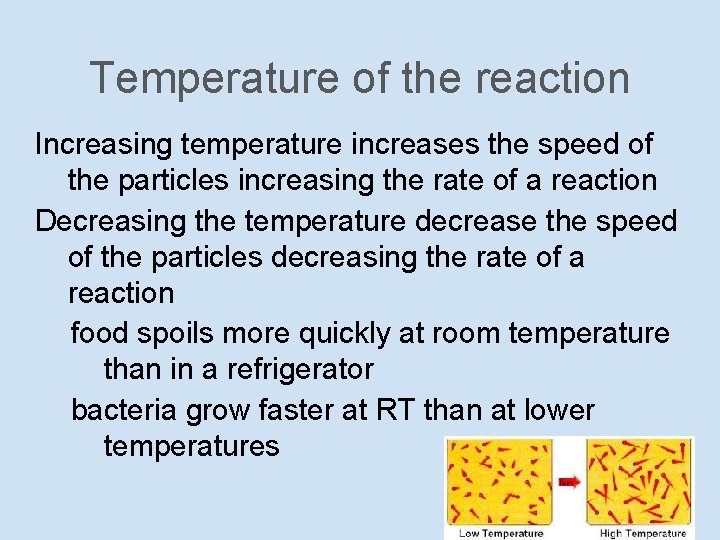
Temperature of the reaction Increasing temperature increases the speed of the particles increasing the rate of a reaction Decreasing the temperature decrease the speed of the particles decreasing the rate of a reaction food spoils more quickly at room temperature than in a refrigerator bacteria grow faster at RT than at lower temperatures

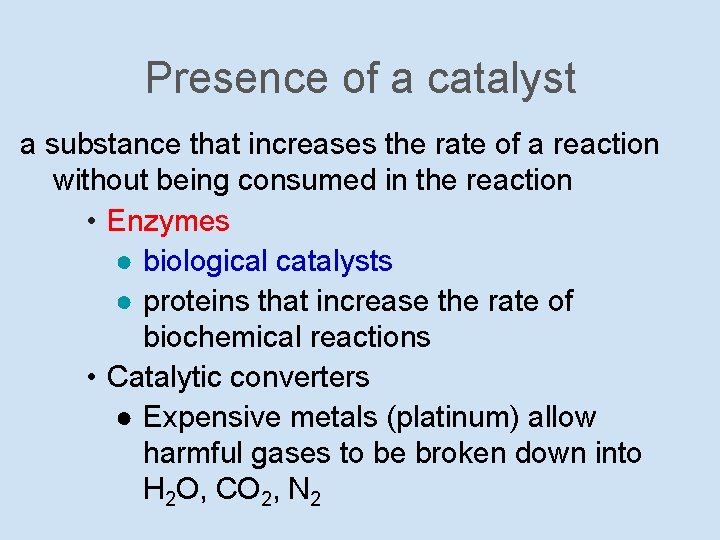
Presence of a catalyst a substance that increases the rate of a reaction without being consumed in the reaction • Enzymes ● biological catalysts ● proteins that increase the rate of biochemical reactions • Catalytic converters ● Expensive metals (platinum) allow harmful gases to be broken down into H 2 O, CO 2, N 2

Surface Area as surface area increases the rate of reaction generally increases Ex: A chunk of iron rusting releases heat VERY SLOWLY. Small amount of heat Iron rusting in a hand warmer releases heat VERY QUICKLY LARGE AMOUNT OF HEAT

Question ➢ What is not something that affects reaction rate? : A. Orientation B. Presence of a catalyst C. Temperature D. Concentration of reactants

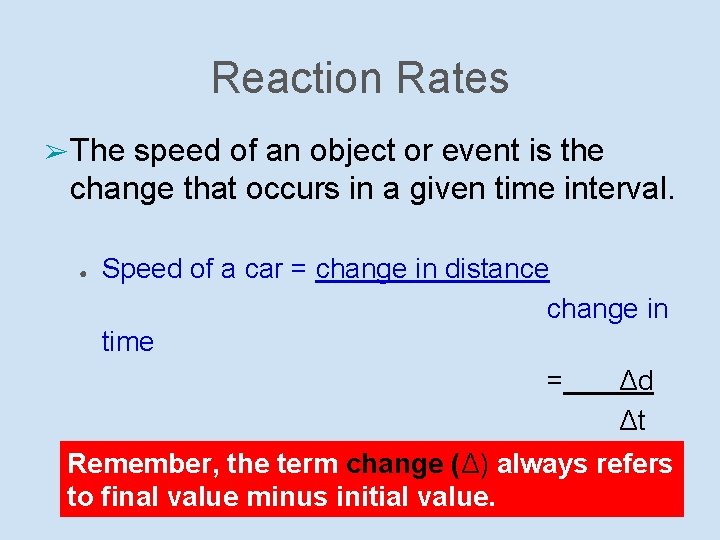
Reaction Rates ➢ The speed of an object or event is the change that occurs in a given time interval. ● Speed of a car = change in distance change in time = Δd Δt Remember, the term change (Δ) always refers to final value minus initial value.

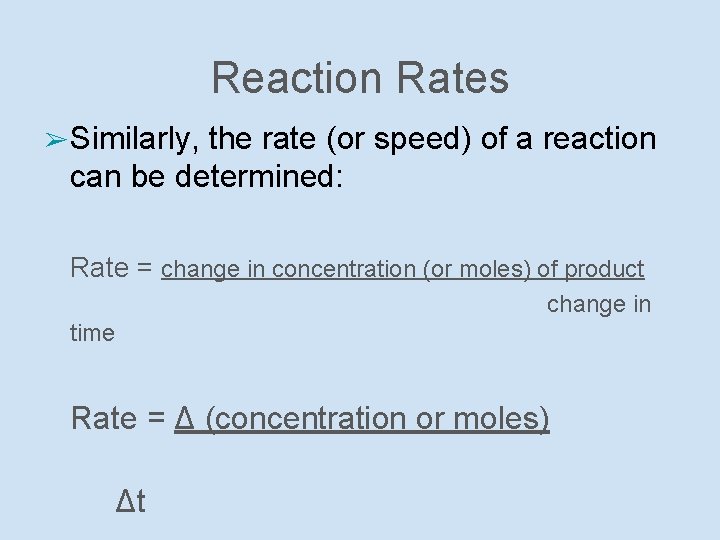
Reaction Rates ➢ Similarly, the rate (or speed) of a reaction can be determined: Rate = change in concentration (or moles) of product change in time Rate = Δ (concentration or moles) Δt

Question ➢ How is reaction rate measured? a. Speed/Distance b. Distance/time c. Mols of product/mols of reactant d. Concentration/time

Comprehension check 1. What 2 factors of collision theory influence a reaction? 1. Pick one of the variables that impacts rate of a reaction. Explain how changing that variable impacts the rate of a reaction
 Proton capture equation
Proton capture equation A rate is a ratio
A rate is a ratio Equivalent ratios
Equivalent ratios Ratios rates and unit rates
Ratios rates and unit rates Ratios rates and unit rates
Ratios rates and unit rates M<1
M<1 Mini unit reaction rates and equilibrium
Mini unit reaction rates and equilibrium Mini unit reaction rates and equilibrium
Mini unit reaction rates and equilibrium Reaction rates and equilibrium worksheet answers chapter 19
Reaction rates and equilibrium worksheet answers chapter 19 Section 4 reaction rates and equilibrium
Section 4 reaction rates and equilibrium Chapter 18 reaction rates and equilibrium answer key
Chapter 18 reaction rates and equilibrium answer key Chapter 18 reaction rates and equilibrium
Chapter 18 reaction rates and equilibrium Expressing reaction rates
Expressing reaction rates Reaction rate
Reaction rate Expressing reaction rates
Expressing reaction rates Did a chemical reaction occur
Did a chemical reaction occur Ictahedron
Ictahedron What is used up in and stops a chemical reaction?
What is used up in and stops a chemical reaction? Chemical reactions of copper and percent yield
Chemical reactions of copper and percent yield Chapter 7 review chemical formulas and chemical compounds
Chapter 7 review chemical formulas and chemical compounds