Atomic Theory Chem 11 Daltons Atomic Theory 1


- Slides: 15

Atomic Theory Chem. 11

Dalton’s Atomic Theory • 1 All matter is made up of invisible particles called atoms • 2 All atoms of one element are identical. The atoms of any one element are different from those of all other element. • 3 Chemical change is the union or separation of atoms • 4 Atoms combine in small whole number ratios to form compounds.

Thomson’s Model • Joseph Thomson realized that the accepted model, of his time, did not take into account electrons and protons. • Thomson created a new model, known as the “plum pudding atom. ”

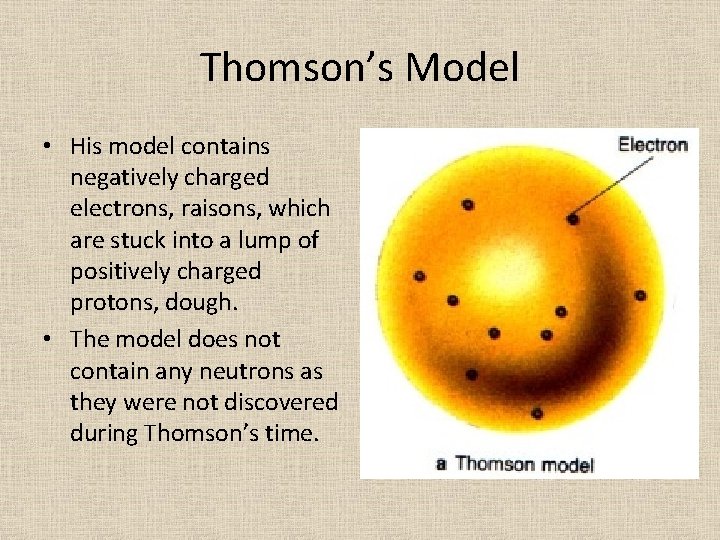
Thomson’s Model • His model contains negatively charged electrons, raisons, which are stuck into a lump of positively charged protons, dough. • The model does not contain any neutrons as they were not discovered during Thomson’s time.

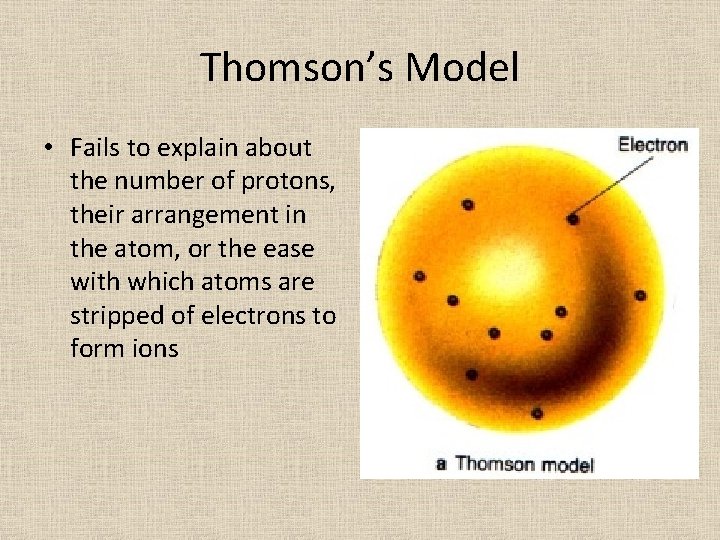
Thomson’s Model • Fails to explain about the number of protons, their arrangement in the atom, or the ease with which atoms are stripped of electrons to form ions

Rutherford’s Model • Ernest Rutherford performed the Gold Foil experiment, which led to his discovery of the nucleus • Rutherford proposed the nuclear atom in which electrons surround a dense nucleus.

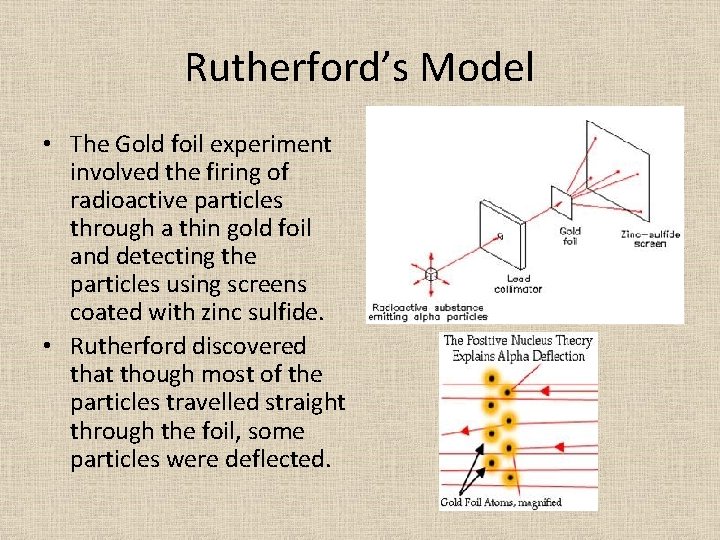
Rutherford’s Model • The Gold foil experiment involved the firing of radioactive particles through a thin gold foil and detecting the particles using screens coated with zinc sulfide. • Rutherford discovered that though most of the particles travelled straight through the foil, some particles were deflected.

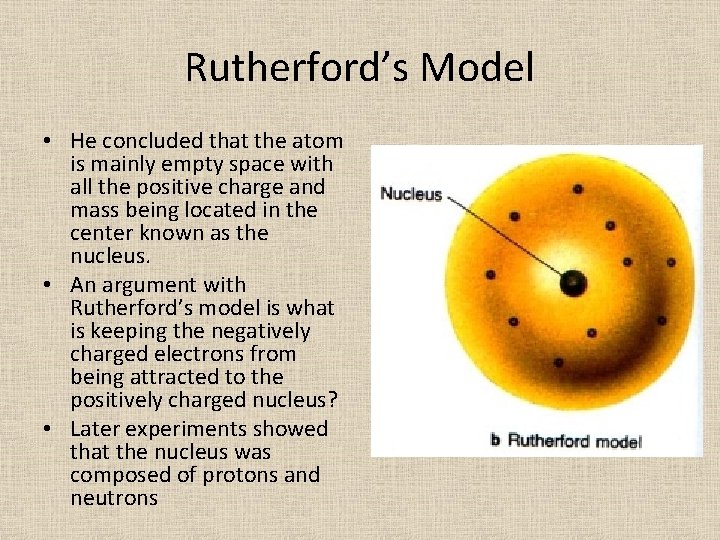
Rutherford’s Model • He concluded that the atom is mainly empty space with all the positive charge and mass being located in the center known as the nucleus. • An argument with Rutherford’s model is what is keeping the negatively charged electrons from being attracted to the positively charged nucleus? • Later experiments showed that the nucleus was composed of protons and neutrons

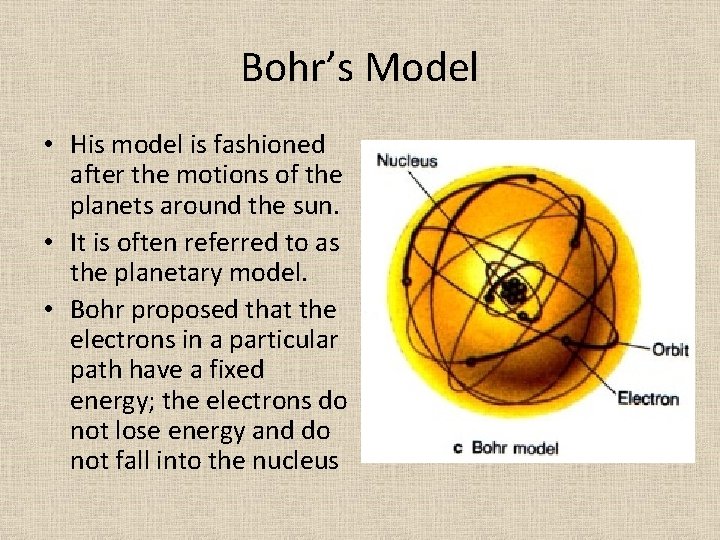
Bohr’s Model • Niels Bohr developed a new atomic model to answer the question of why electrons do not collapse into the nucleus in Rutherford’s model. • He proposed that electrons are arranged in concentric circular paths, know as orbits, around the nucleus.

Bohr’s Model • His model is fashioned after the motions of the planets around the sun. • It is often referred to as the planetary model. • Bohr proposed that the electrons in a particular path have a fixed energy; the electrons do not lose energy and do not fall into the nucleus

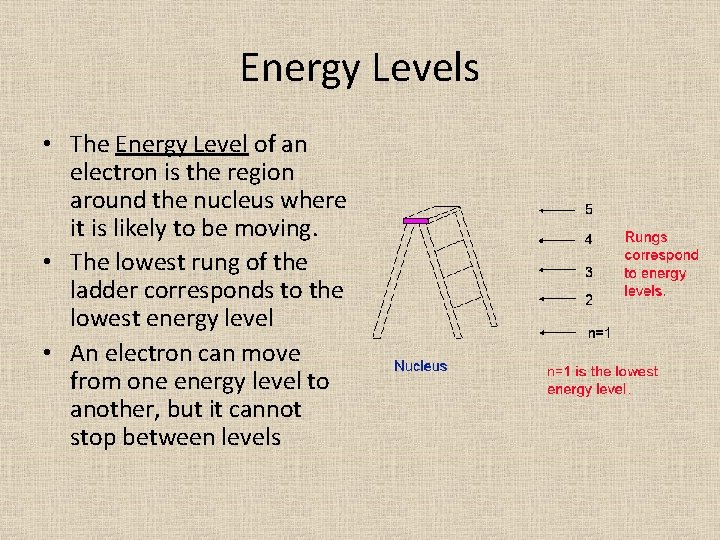
Energy Levels • The Energy Level of an electron is the region around the nucleus where it is likely to be moving. • The lowest rung of the ladder corresponds to the lowest energy level • An electron can move from one energy level to another, but it cannot stop between levels

Energy Levels • An electron must lose or gain just the right amount of energy to move from one energy level to another. • The amount of energy gained or lost by every electron is not the same. • The energy levels in an atom are not equally spaced.

Energy Levels • A Quantum of energy is the amount of energy required to move an electron from its present energy level to the next higher level. • The energies of electrons are said to be quantized • In general, the higher an electron is placed on the energy ladder, the larger the diameter of the circular path of that electron

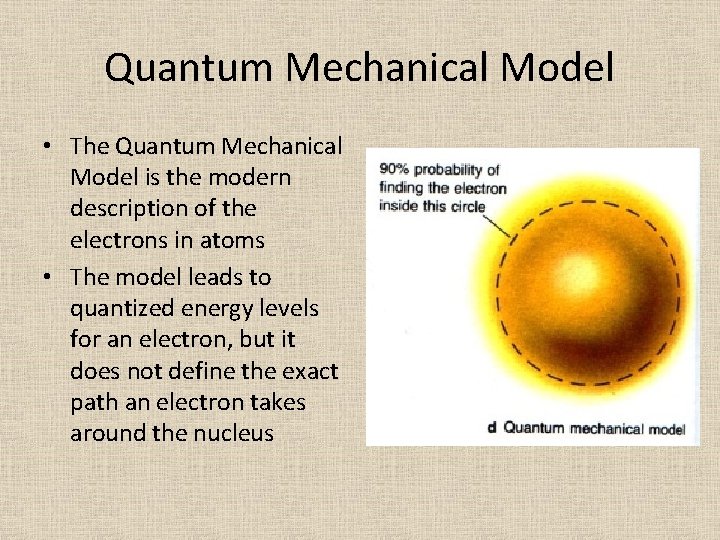
Quantum Mechanical Model • The Quantum Mechanical Model is the modern description of the electrons in atoms • The model leads to quantized energy levels for an electron, but it does not define the exact path an electron takes around the nucleus

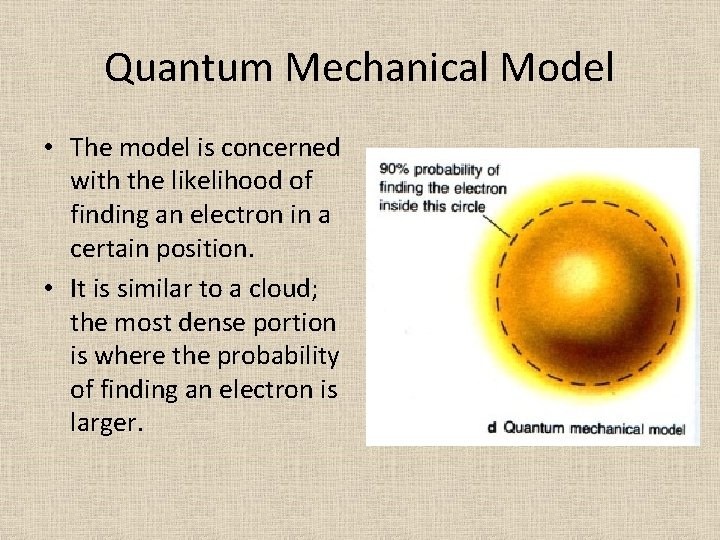
Quantum Mechanical Model • The model is concerned with the likelihood of finding an electron in a certain position. • It is similar to a cloud; the most dense portion is where the probability of finding an electron is larger.