The Evolution of the Atomic Model Daltons Atomic



- Slides: 10

The Evolution of the Atomic Model

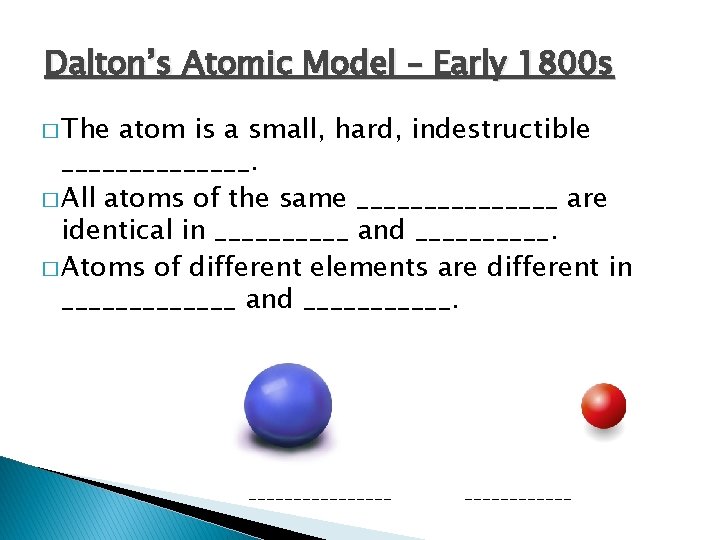
Dalton’s Atomic Model – Early 1800 s � The atom is a small, hard, indestructible _______. � All atoms of the same ________ are identical in _____ and _____. � Atoms of different elements are different in _______ and ________

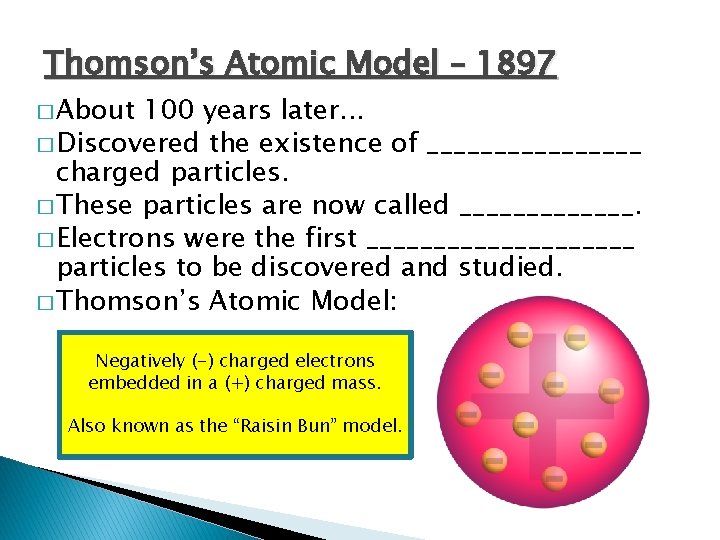
Thomson’s Atomic Model – 1897 � About 100 years later. . . � Discovered the existence of ________ charged particles. � These particles are now called _______. � Electrons were the first __________ particles to be discovered and studied. � Thomson’s Atomic Model: Negatively (-) charged electrons embedded in a (+) charged mass. Also known as the “Raisin Bun” model.

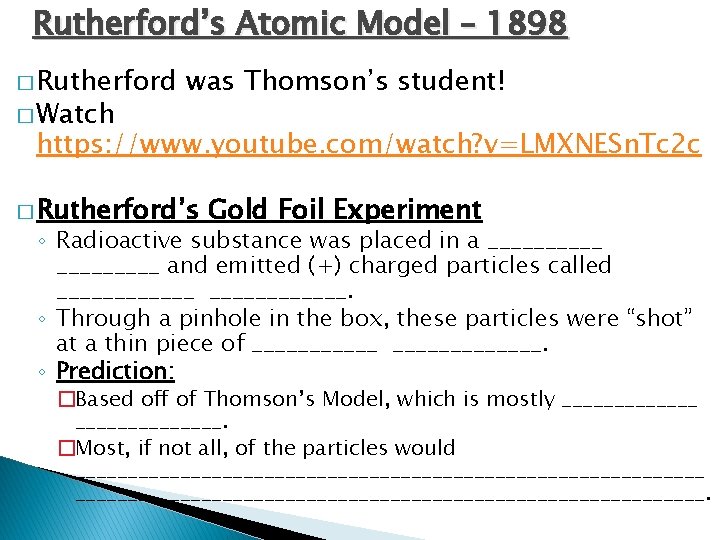
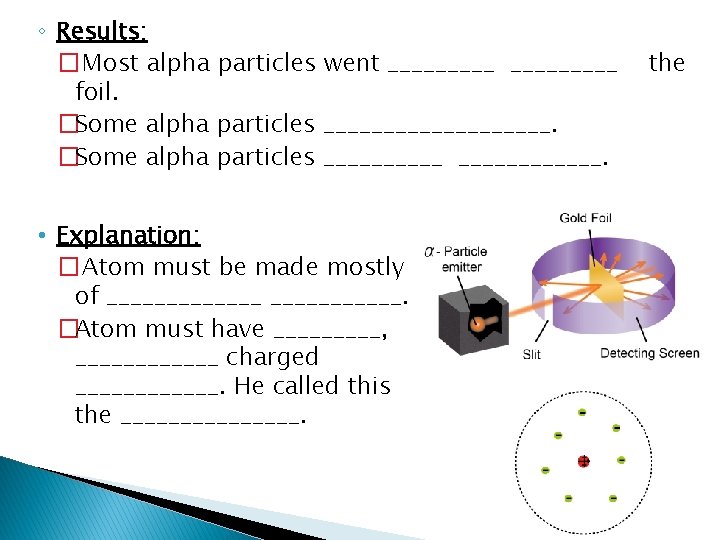
Rutherford’s Atomic Model – 1898 � Rutherford � Watch was Thomson’s student! https: //www. youtube. com/watch? v=LMXNESn. Tc 2 c � Rutherford’s Gold Foil Experiment ◦ Radioactive substance was placed in a _____ and emitted (+) charged particles called ____________. ◦ Through a pinhole in the box, these particles were “shot” at a thin piece of _____________. ◦ Prediction: �Based off of Thomson’s Model, which is mostly ______________. �Most, if not all, of the particles would ____________________________________________________________.

◦ Results: � Most alpha particles went _________ foil. �Some alpha particles ____________. • Explanation: � Atom must be made mostly of _______. �Atom must have _____, ______ charged ______. He called this the ________. the

BUT WAIT. . If the nucleus is positively charged, and electrons are negatively charged, shouldn’t they attract each other? Shouldn’t the electrons just end up spiralling into the nucleus? !

Bohr & Energy Levels - 1912 � Determined that there are only a few levels in which electrons can exist. � Proposed the idea existence of the atomic energy level. � Electrons can jump from a lower level to a higher level when they ____ a ______ of energy (electric current, spark, heat, or light). � Electrons can drop from a higher level to a lower level when they ____ a ______ of energy (released in the form of light). � Electrons can not exist in between the allowed energy levels.

James Chadwick - 1932 � Eventually, after many years of work by several scientists, the positive charge in the nucleus was caused by subatomic particles that they called _________. � Scientific evidence indicated there was more than just one type of particle in the nucleus. � For example, Rutherford knew that the mass of the nucleus was more than the mass of the protons alone. � James Chadwick (a former co-worker of Rutherford’s), showed experimentally that neutral particles, now called _______, help make up the nuclei of most atoms.

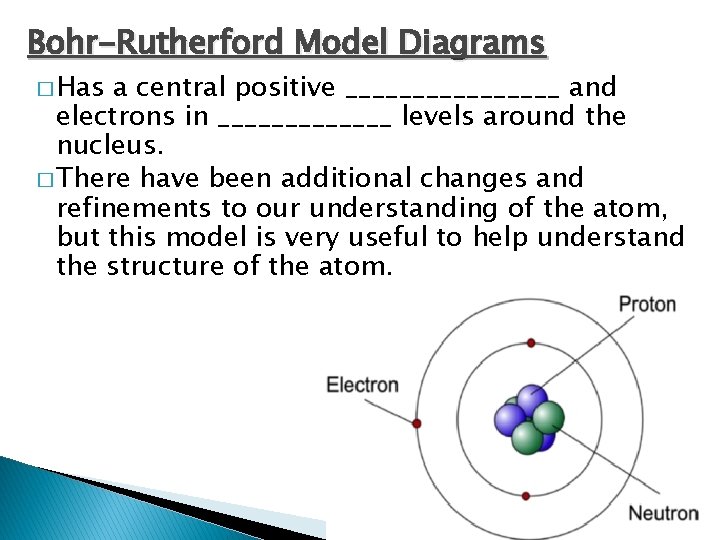
Bohr-Rutherford Model Diagrams � Has a central positive ________ and electrons in _______ levels around the nucleus. � There have been additional changes and refinements to our understanding of the atom, but this model is very useful to help understand the structure of the atom.

Atomic Model Summary Briefly describe each model, and sketch a diagram below each description. Dalton Thomson Rutherford Bohr-Rutherford