Chapter 2 Elements Atoms Daltons Atomic Theory An














- Slides: 26

Chapter 2 Elements & Atoms

Dalton’s Atomic Theory • An element is composed of tiny particles called atoms. All atoms of a given element show the same chemical properties. • Atoms of different elements have different properties. • Compounds are formed when atoms of two or more elements combine. In a given compound, the relative number of atoms of each kind are definite and constant. • In an ordinary chemical reaction, no atom of any element disappears or is changed into an atom of another element. Chemical reactions involve changing the way in which the atoms are joined together. • The same elements can be combined to form different compounds by combining the elements in different proportion.

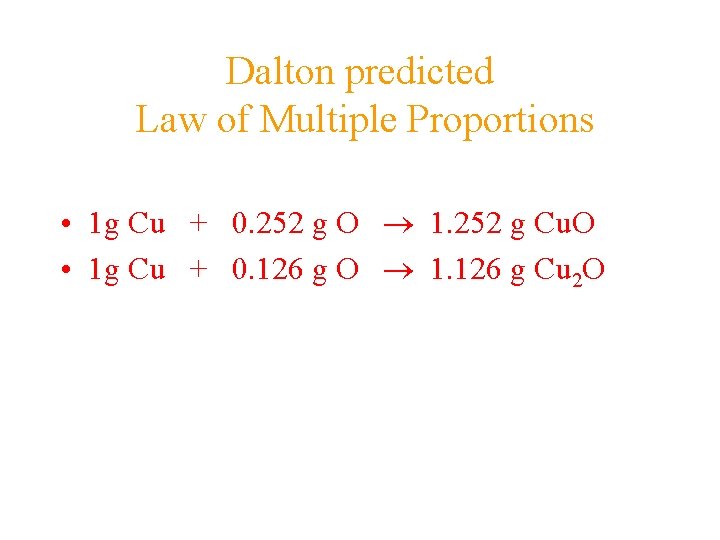
Dalton predicted Law of Multiple Proportions • 1 g Cu + 0. 252 g O 1. 252 g Cu. O • 1 g Cu + 0. 126 g O 1. 126 g Cu 2 O

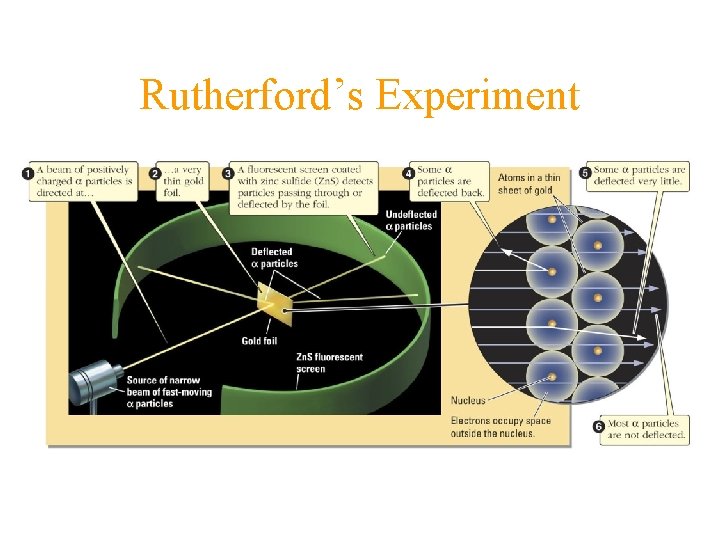
Rutherford’s Experiment

Rutherford’s Model of the Atom • atom is composed mainly of vacant space • all the positive charge and most of the mass is in a small area called the nucleus • electrons are in the electron cloud surrounding the nucleus

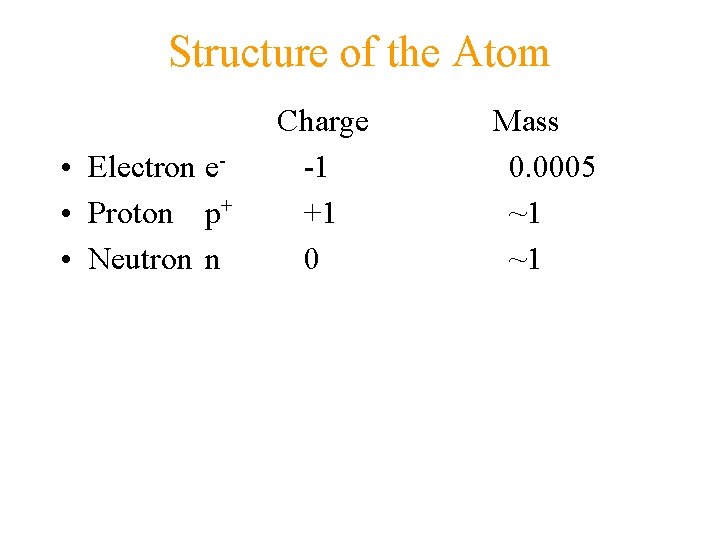
Structure of the Atom • Electron e • Proton p+ • Neutron n Charge -1 +1 0 Mass 0. 0005 ~1 ~1

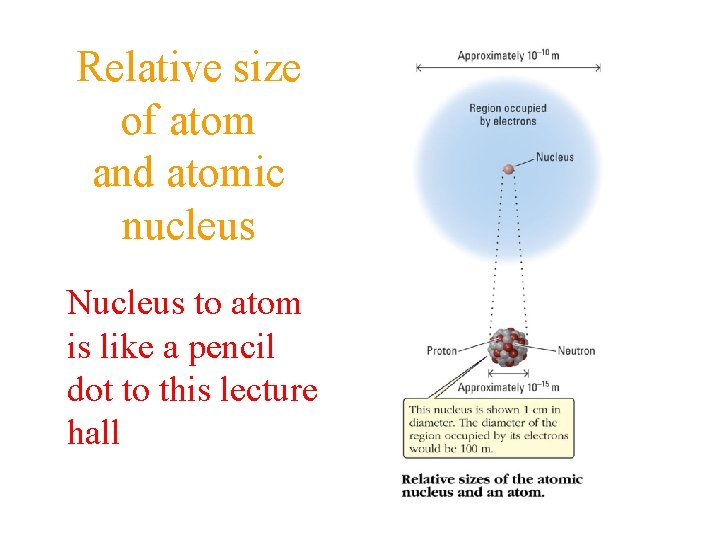
Relative size of atom and atomic nucleus Nucleus to atom is like a pencil dot to this lecture hall

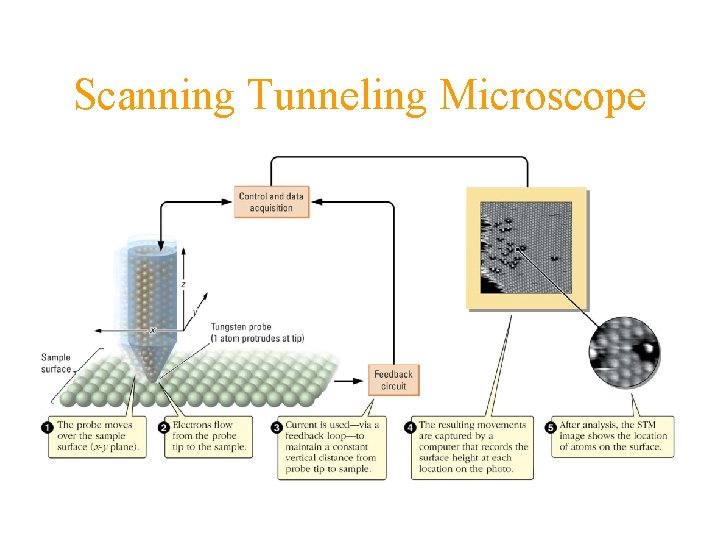
Scanning Tunneling Microscope


Atomic number, Z • the number of protons in the nucleus • the number of electrons in a neutral atom • the integer on the periodic table for each element

Mass Number, A • the sum of the number of protons and neutrons in the nucleus • Approximately the mass of an atom

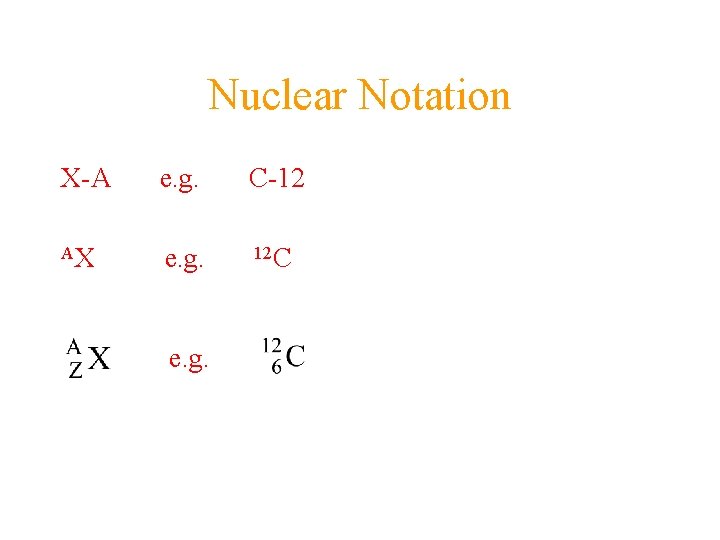
Nuclear Notation X-A e. g. C-12 AX e. g. 12 C e. g.

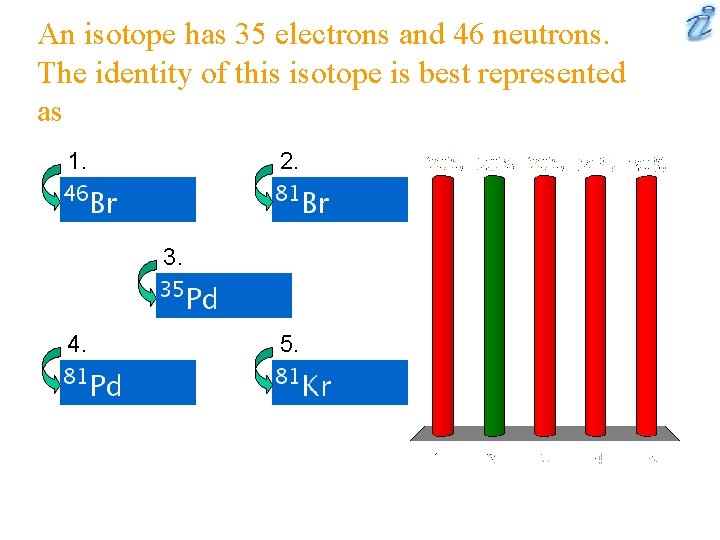
An isotope has 35 electrons and 46 neutrons. The identity of this isotope is best represented as 1. 2. 3. 4. 5.

Masses of Atoms Carbon-12 Scale Masses of the atoms are compared to the mass of C-12 isotope having a mass of 12. 0000

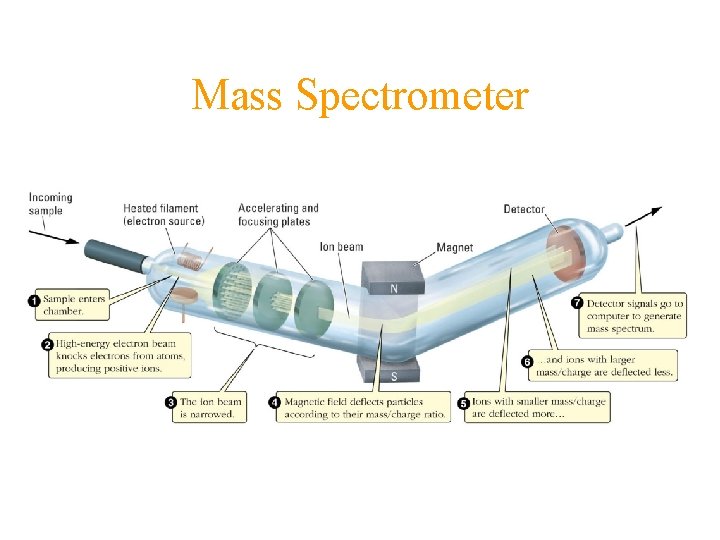
Mass Spectrometer

Atomic Masses and Isotopic Abundances natural atomic masses = sum[(atomic mass of isotope) (fractional isotopic abundance)] The average mass of the naturally occurring isotopes Cl 34. 969 x 0. 7577+36. 966 x 0. 2423=35. 453 amu

1. 2. 3. 4.

The Mole • a unit of measurement, quantity of matter present • Avogadro’s Number 6. 022 1023 particles per mole • Latin for “pile” • 1 C-12 atom has mass 12 amu • 1 mole C-12 has mass 12 g

• Avogadro’s number converts between moles and number of particles • Molar mass converts between moles and mass

• You are given a 1 carat diamond, how many carbon atoms does it contain (1 carat=0. 2 g) • 1 carat C x 0. 2 g/1 carat = 0. 2 g C • 0. 2 g C x 1 mol C/12. 01 g C = 0. 01665 mol C • 0. 01665 mol C x 6. 022 x 1023 atoms C/1 mol C =1. 003 x 1022 C atoms

Development of Periodic Table Mendeleev • 1869 - Periodic Law • allowed him to predict properties of unknown elements • the elements are arranged according to increasing atomic weights • Missing elements: 44, 68, 72, & 100 amu

Predicted Properties of Ekasilicon

Modern Periodic Table Moseley, Henry Gwyn Jeffreys 1887– 1915, English physicist. • studied the relations among spectra of different elements. • concluded that the atomic number is equal to the charge on the nucleus based on the x-ray spectra emitted by the element. • explained discrepancies in Mendeleev’s Periodic Law.

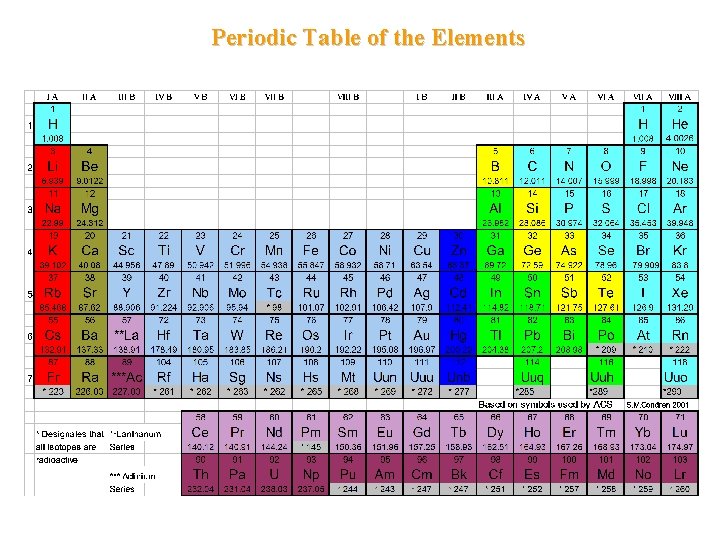
Periodic Table of the Elements

Family Names Group IA alkali metals Group IIA alkaline earth metals Group VIIA halogens Group VIIIA noble gases transition metals inner transition metals • lanthanum series rare earths • actinium series trans-uranium series

Diatomic Elements • H 2 N 2 O 2 F 2 Cl 2 Br 2 I 2 Memorize • The “gens” • Hydrogen, nitrogen, oxygen, halogens • S 8 and P 4 No need to remember