Chapter 4 Atomic Structure 1 Daltons Atomic Theory












- Slides: 54

Chapter 4 Atomic Structure 1

Dalton’s Atomic Theory (1) Dalton (British) 1803 Ø proposed the atomic theory Ø remained essentially intact up to the present time. 2

Dalton’s Atomic Theory (2) Dalton’s atomic theory. 1. All matters are made up of atoms. 2. Atoms are indivisible. 3. All atoms of 1 element are exactly alike, but are different from atoms of other elements. 3

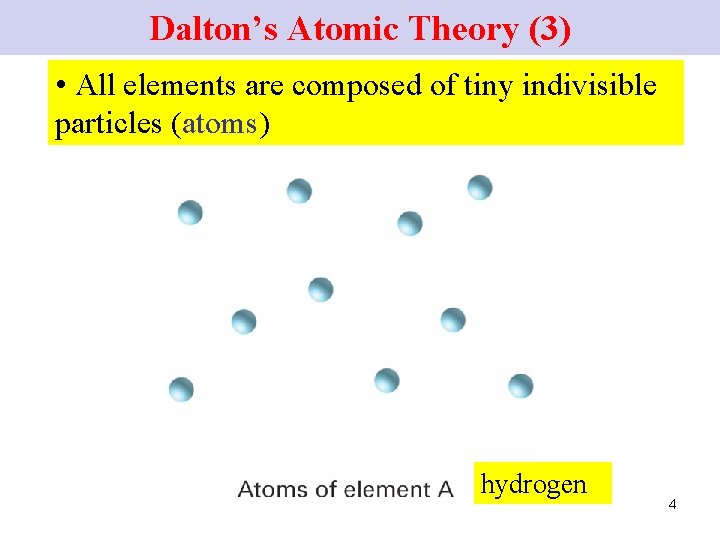
Dalton’s Atomic Theory (3) • All elements are composed of tiny indivisible particles (atoms) hydrogen 4

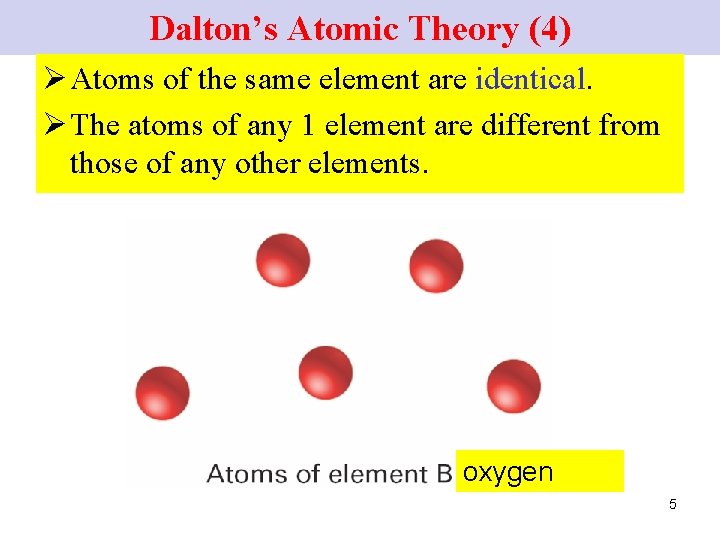
Dalton’s Atomic Theory (4) Ø Atoms of the same element are identical. Ø The atoms of any 1 element are different from those of any other elements. oxygen 5

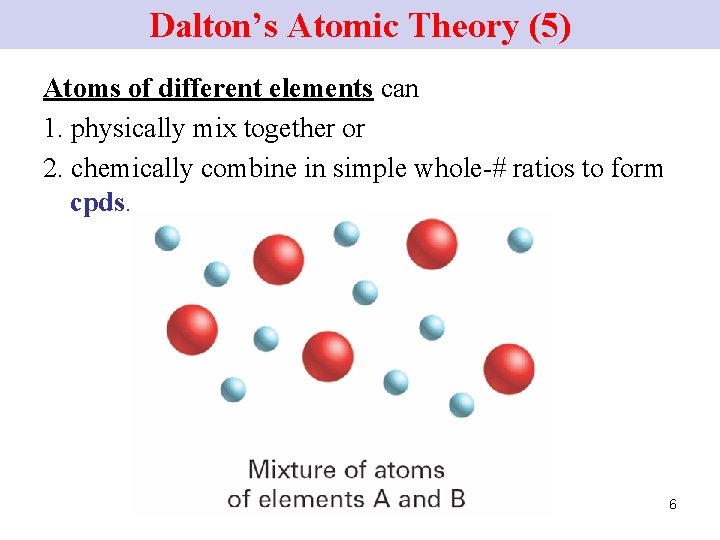
Dalton’s Atomic Theory (5) Atoms of different elements can 1. physically mix together or 2. chemically combine in simple whole-# ratios to form cpds. 6

Dalton’s Atomic Theory (6) ØChem rxns occur when atoms are separated, joined, or rearranged. ØAtoms of 1 element are never changed into atoms of another element in a chem rxn. 7

Cathode Ray Expt (1) • Because of Dalton’s atomic theory, most scientists in the 1800 s believed that the atom was like a tiny solid ball. • In 1897, Thomson (British), discovered that this solid-ball model was not accurate. 8

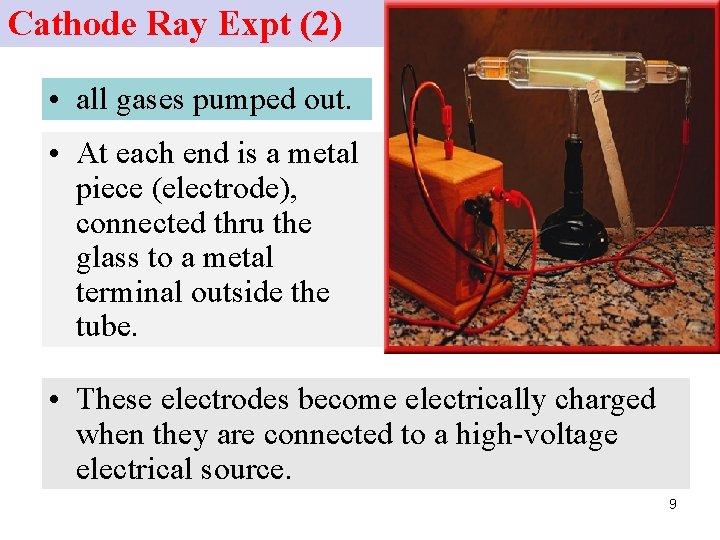
Cathode Ray Expt (2) • all gases pumped out. • At each end is a metal piece (electrode), connected thru the glass to a metal terminal outside the tube. • These electrodes become electrically charged when they are connected to a high-voltage electrical source. 9

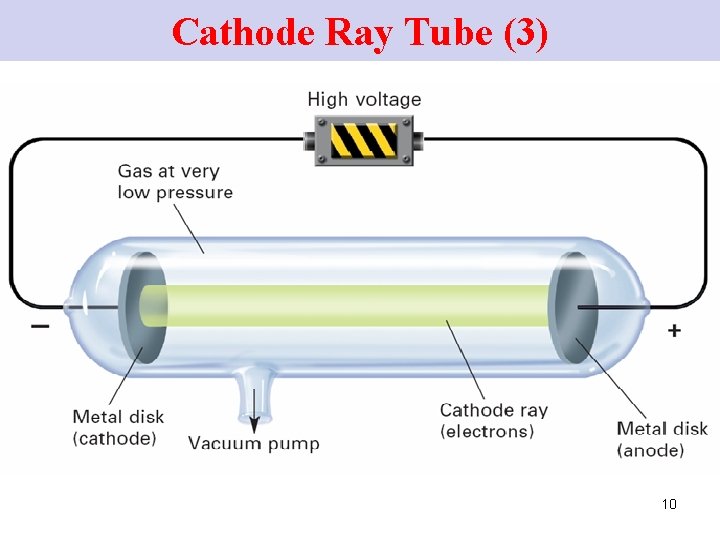
Cathode Ray Tube (3) 10

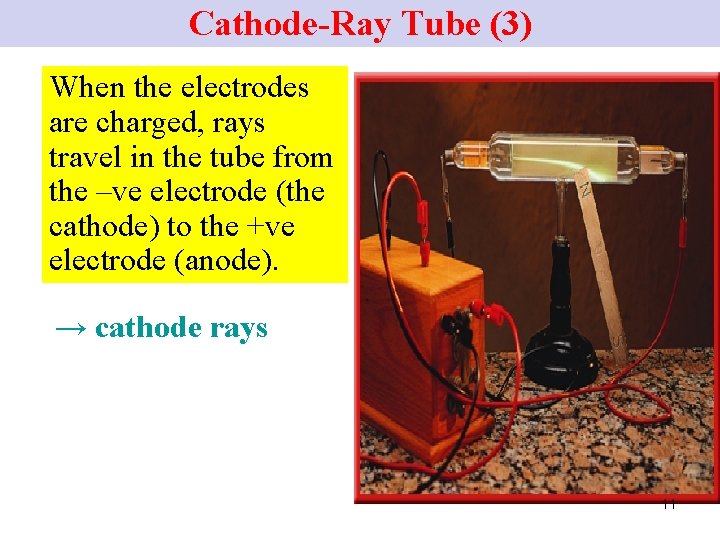
Cathode-Ray Tube (3) When the electrodes are charged, rays travel in the tube from the –ve electrode (the cathode) to the +ve electrode (anode). → cathode rays 11

Cathode Ray Expt (4) A cathode ray is deflected by a magnet. 12

Thomson’s Finding Ø Cathode rays bent toward a +vely charged plate and away from a –vely charged plate. Background: Objects with like charges repel each other, and objects with unlike charges attract each other. 13

Thomson’s Conclusions (1) Conclusion (Cathode Ray Expt. ) Ø that cathode rays are made up of invisible, – vely charged particles -- electrons. Ø These e- had to come from the matter (atoms) of the +ve electrode. 14

Thomson’s Conclusion (2) Ø atoms were not just neutral spheres, but Ø composed of electrically charged particles. Ø Matter is not –vely charged, so atoms can’t be –vely charged either. If atoms contained –vely charged particles, then they must also contain = # of +vely charged particles. 15

Thomson’s Conclusions (4) Ø Thomson concluded that a cathode ray is a stream of e-. e- are parts of the atoms of all elements. 16

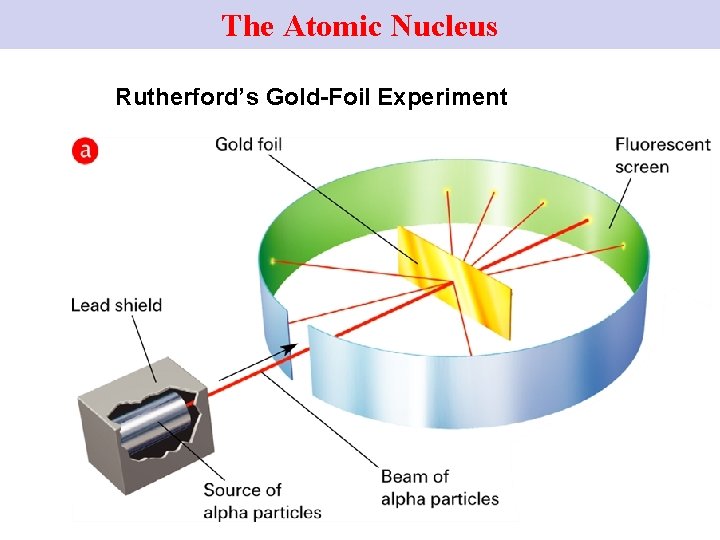
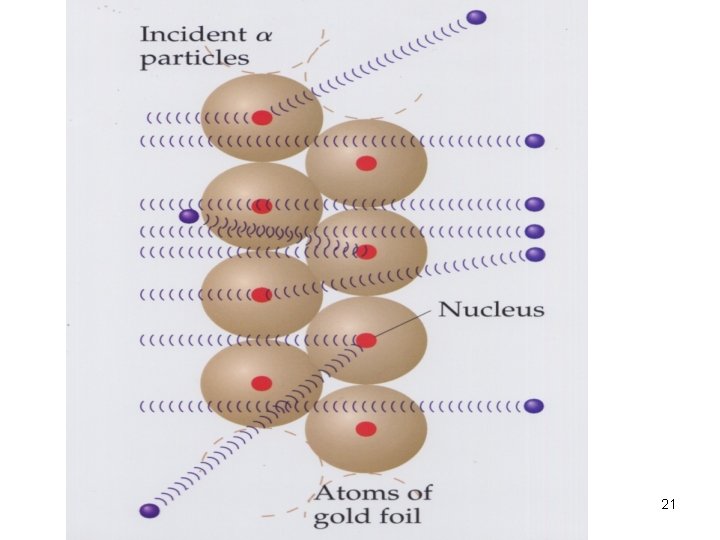
Rutherford’s Gold Foil Experiment (1) • In 1909, Rutherford, (British), … • Conducted the Gold Foil Expt. 17

Rutherford’s Gold Foil Expt (2) Rutherford directed a narrow beam of alpha particles (+vely charged) at a very thin sheet of gold foil. 18

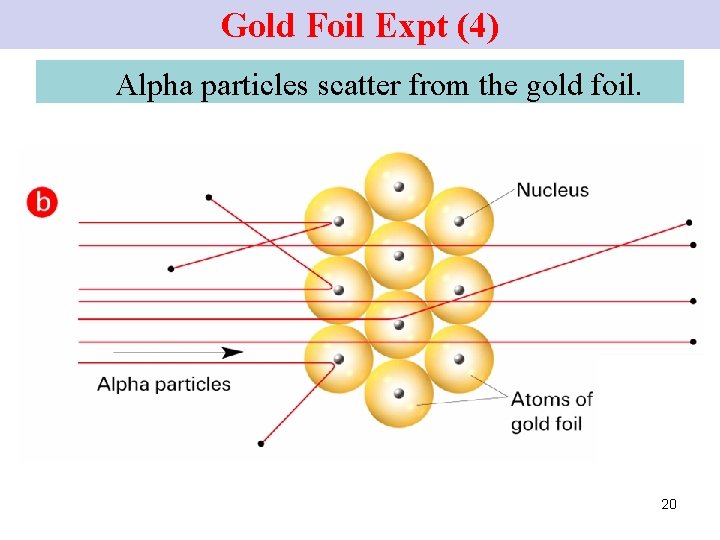
The Atomic Nucleus Rutherford’s Gold-Foil Experiment

Gold Foil Expt (4) Alpha particles scatter from the gold foil. 20

21

Conclusions of Gold Foil expt (1)) > 99% of particles passed thru the foil in a straight line, …. the atom is nearly all empty space. charged (repel alpha particles) (2) some particles got deflected, they should have hit the hard cores of the atoms which are +vely (3) So few particles (<1%) were deflected, …the central cores must be very small. 22

Quick-write How could Rutherford conclude that the nucleus is very small compared with the size of the atom? 23

Quick-write • How could Rutherford conclude that the nucleus is positively charged? 24

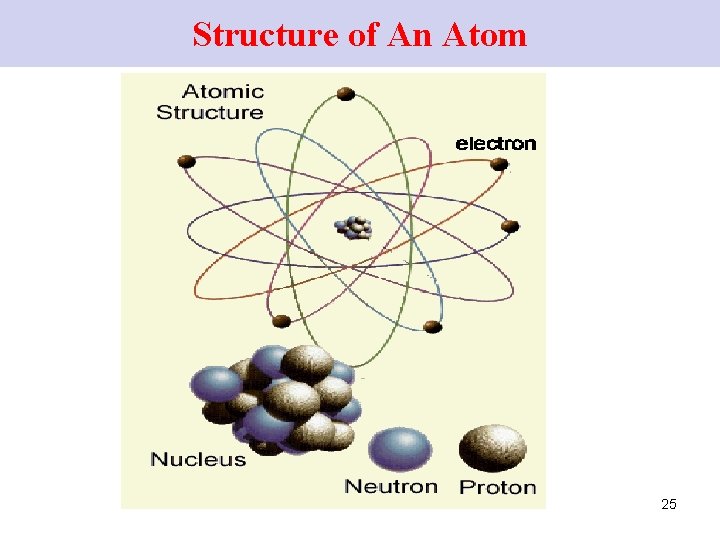
Structure of An Atom 25

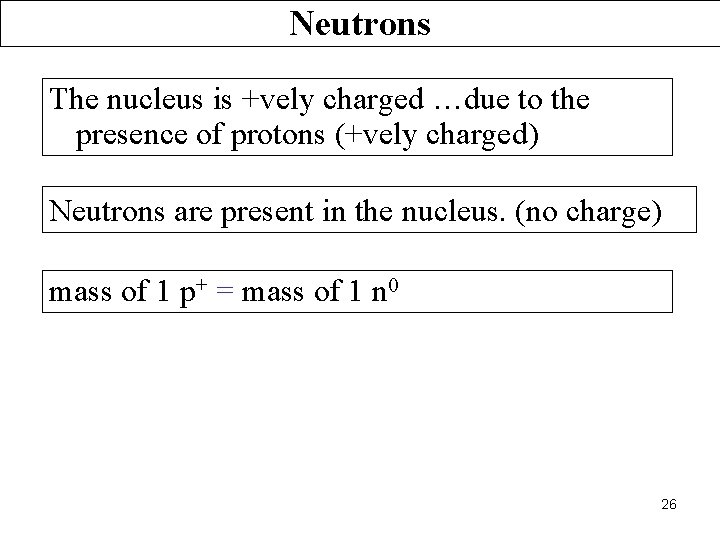
Neutrons The nucleus is +vely charged …due to the presence of protons (+vely charged) Neutrons are present in the nucleus. (no charge) mass of 1 p+ = mass of 1 n 0 26

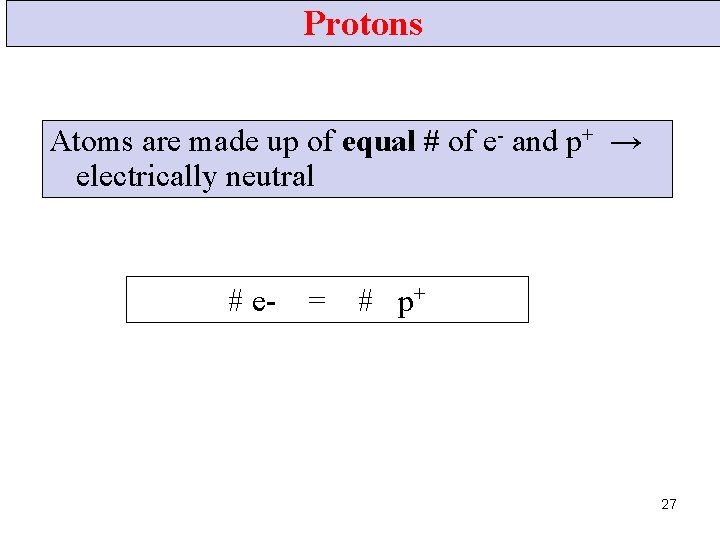
Protons Atoms are made up of equal # of e- and p+ → electrically neutral # e- = # p+ 27

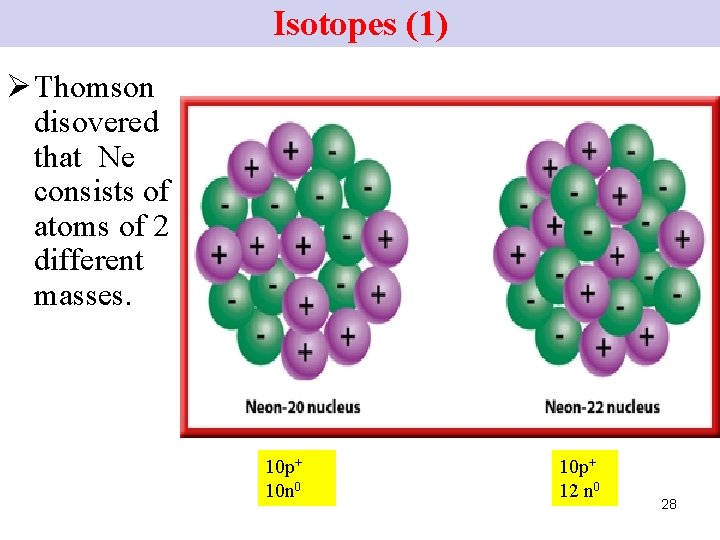
Isotopes (1) Ø Thomson disovered that Ne consists of atoms of 2 different masses. 10 p+ 10 n 0 10 p+ 12 n 0 28

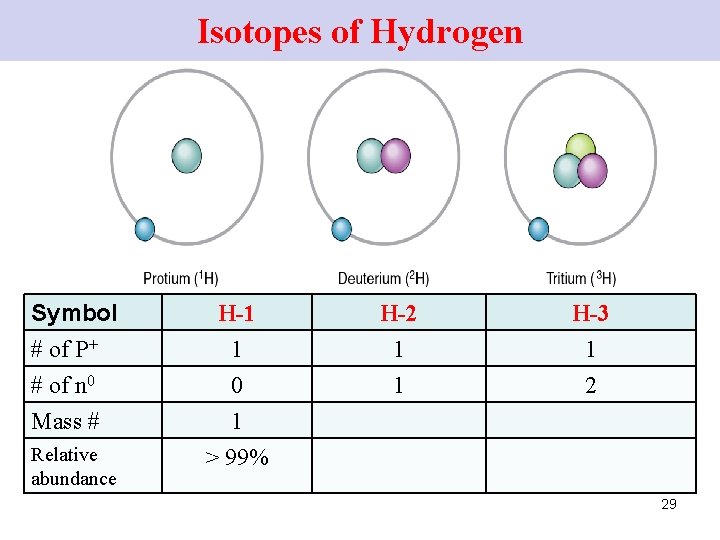
Isotopes of Hydrogen Symbol # of P+ # of n 0 Mass # H-1 1 0 1 Relative abundance > 99% H-2 1 1 H-3 1 2 29

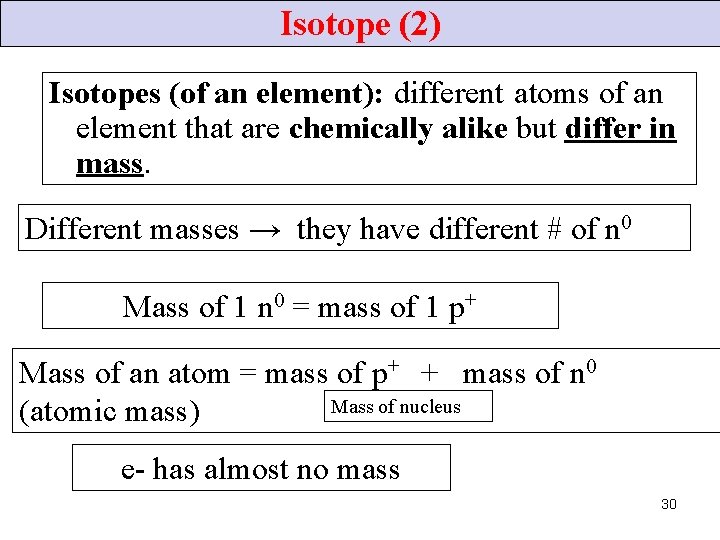
Isotope (2) Isotopes (of an element): different atoms of an element that are chemically alike but differ in mass. Different masses → they have different # of n 0 Mass of 1 n 0 = mass of 1 p+ Mass of an atom = mass of p+ + mass of n 0 Mass of nucleus (atomic mass) e- has almost no mass 30

Quick-write Why different isotopes of the same element have different masses? 31

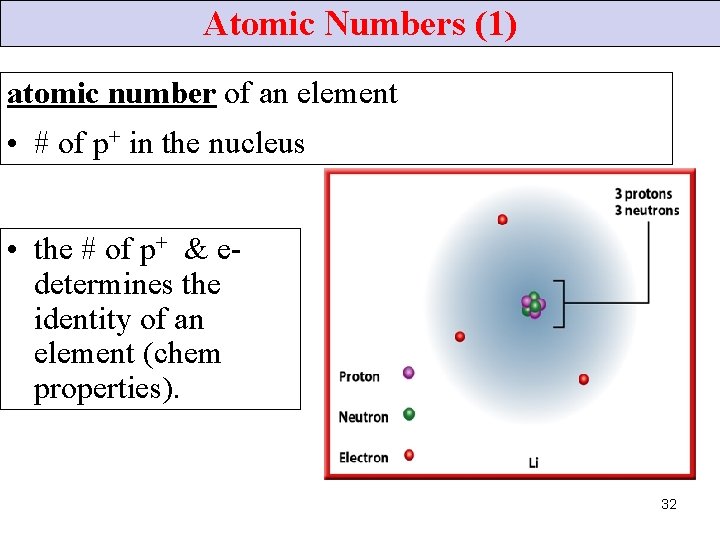
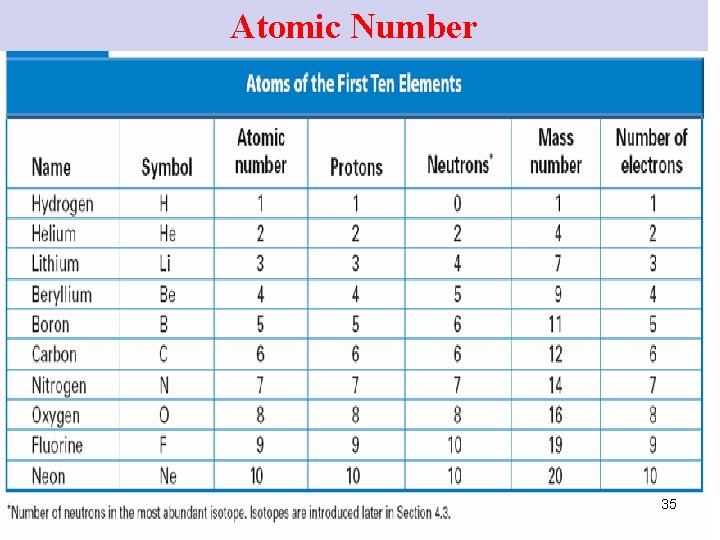
Atomic Numbers (1) atomic number of an element • # of p+ in the nucleus • the # of p+ & edetermines the identity of an element (chem properties). 32

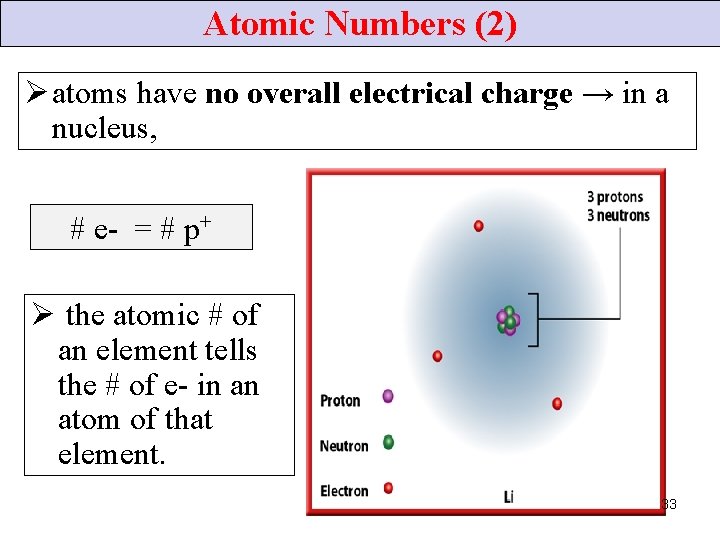
Atomic Numbers (2) Ø atoms have no overall electrical charge → in a nucleus, # e- = # p+ Ø the atomic # of an element tells the # of e- in an atom of that element. 33

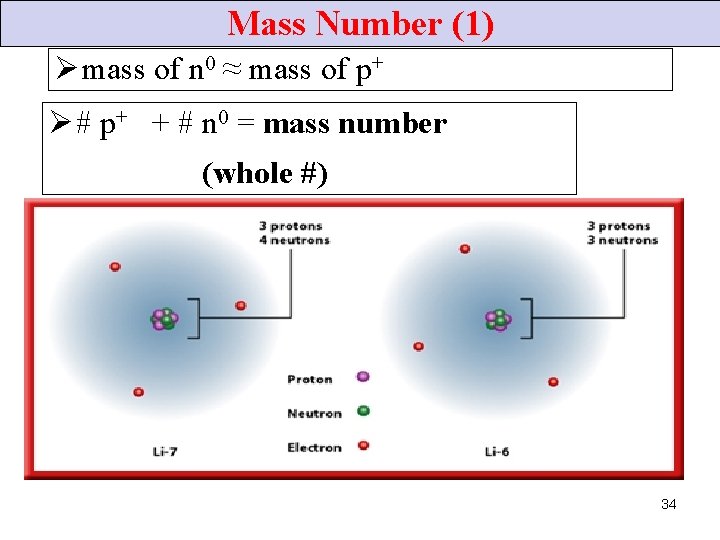
Mass Number (1) Ø mass of n 0 ≈ mass of p+ Ø # p+ + # n 0 = mass number (whole #) 34

Atomic Number 35

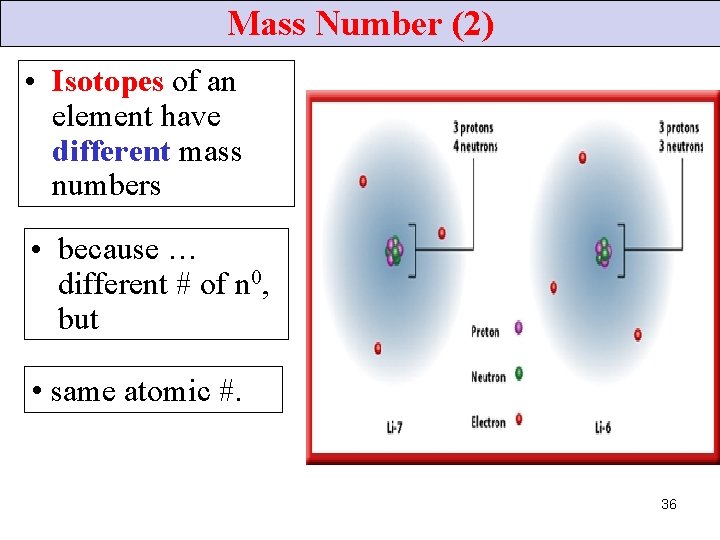
Mass Number (2) • Isotopes of an element have different mass numbers • because … different # of n 0, but • same atomic #. 36

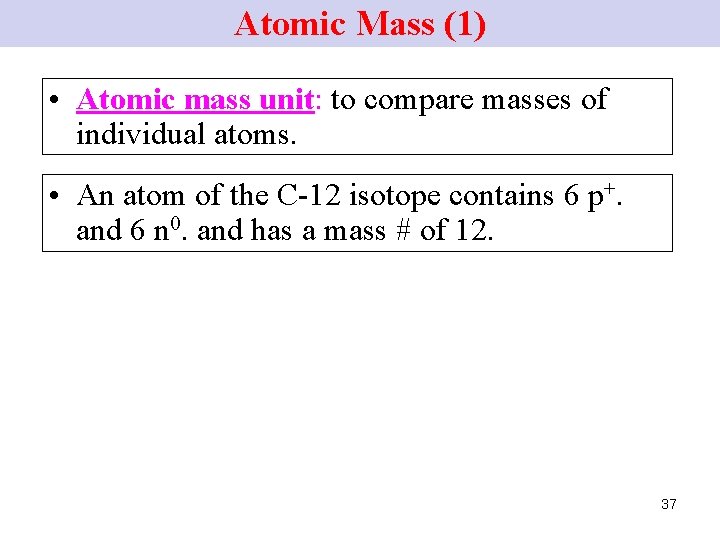
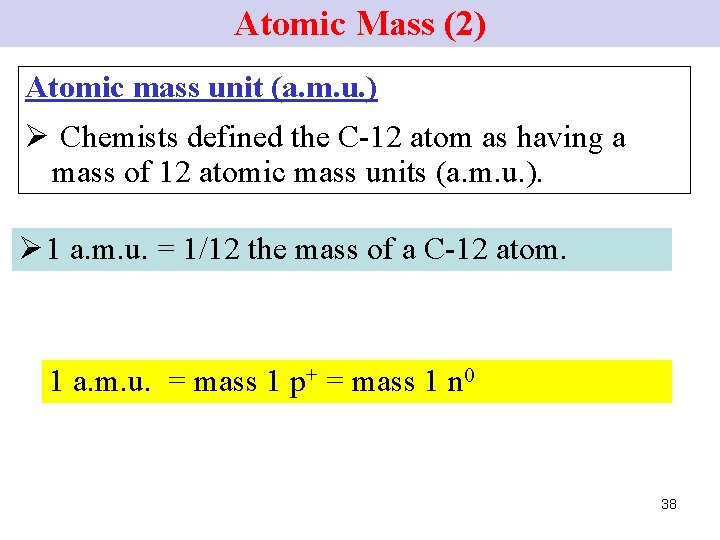
Atomic Mass (1) • Atomic mass unit: to compare masses of individual atoms. • An atom of the C-12 isotope contains 6 p+. and 6 n 0. and has a mass # of 12. 37

Atomic Mass (2) Atomic mass unit (a. m. u. ) Ø Chemists defined the C-12 atom as having a mass of 12 atomic mass units (a. m. u. ). Ø 1 a. m. u. = 1/12 the mass of a C-12 atom. 1 a. m. u. = mass 1 p+ = mass 1 n 0 38

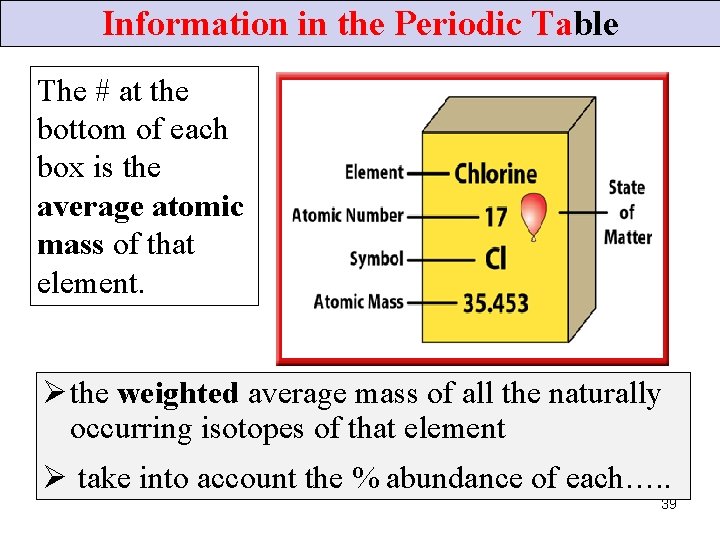
Information in the Periodic Table The # at the bottom of each box is the average atomic mass of that element. Ø the weighted average mass of all the naturally occurring isotopes of that element Ø take into account the % abundance of each…. . 39

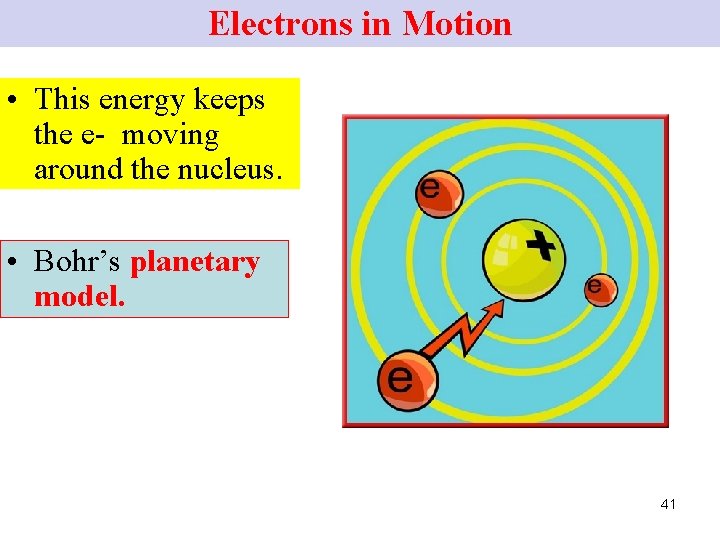
Electrons in Motion Bohr proposed Ø that e- must have enough energy to keep them in constant motion around the nucleus. Ø k. e. Ø energy of motion to overcome the attraction of the nucleus (+ve). 40

Electrons in Motion • This energy keeps the e- moving around the nucleus. • Bohr’s planetary model. 41

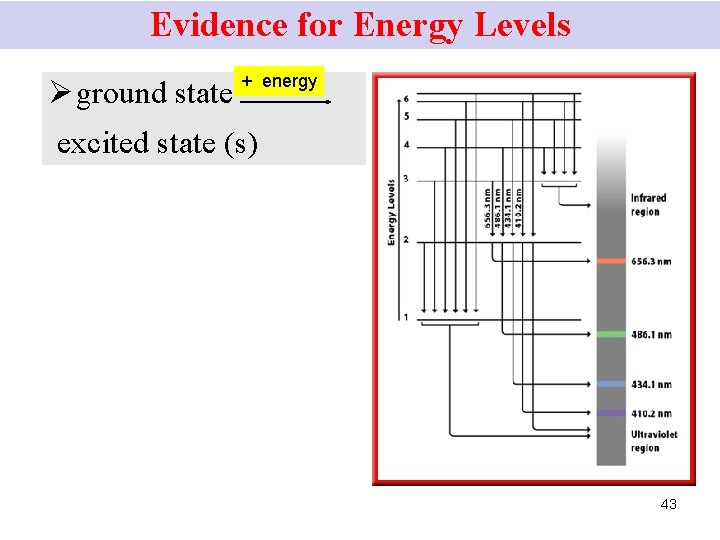
Energy Levels Ground state § e- at the lowest EL Excited state(s) • e- jumps to a higher EL possessing more energy; after absorbing energy such as heat. 42

Evidence for Energy Levels Ø ground state + energy excited state (s) 43

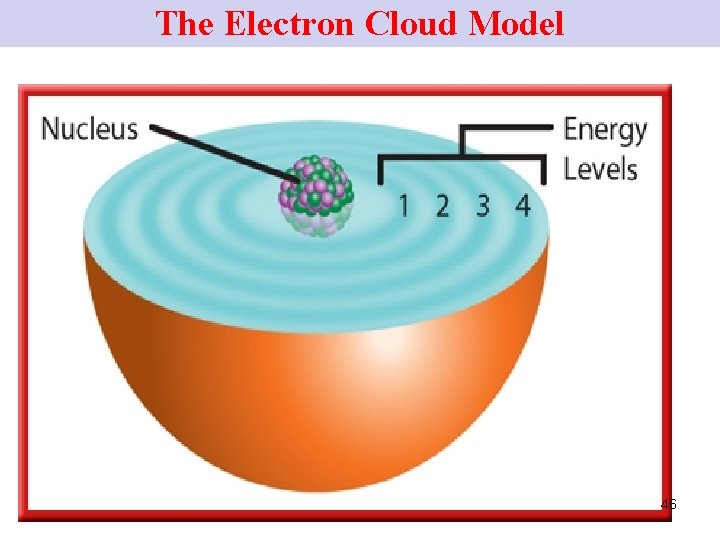
The Electron Cloud Model • scientists today realize that ELs are not neat, planet-like orbits around the nucleus of an atom. • Instead …. spherical regions of space around the nucleus in which e- are most likely to be found. 44

The Electron Cloud Model • e- themselves take up little space but travel rapidly thru the space surrounding the nucleus. • These spherical regions where e- travel may be depicted as clouds around the nucleus. • The space around the nucleus of an atom where the atom’s e- are found is called the e - cloud. 45

The Electron Cloud Model 46

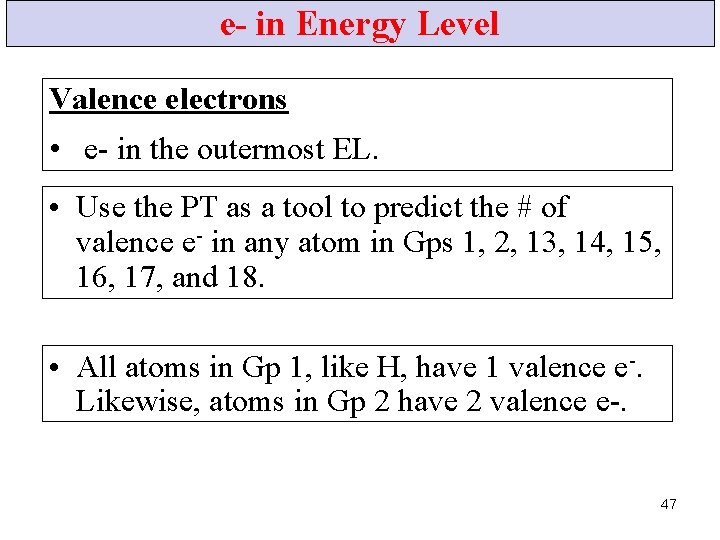
e- in Energy Level Valence electrons • e- in the outermost EL. • Use the PT as a tool to predict the # of valence e- in any atom in Gps 1, 2, 13, 14, 15, 16, 17, and 18. • All atoms in Gp 1, like H, have 1 valence e-. Likewise, atoms in Gp 2 have 2 valence e-. 47

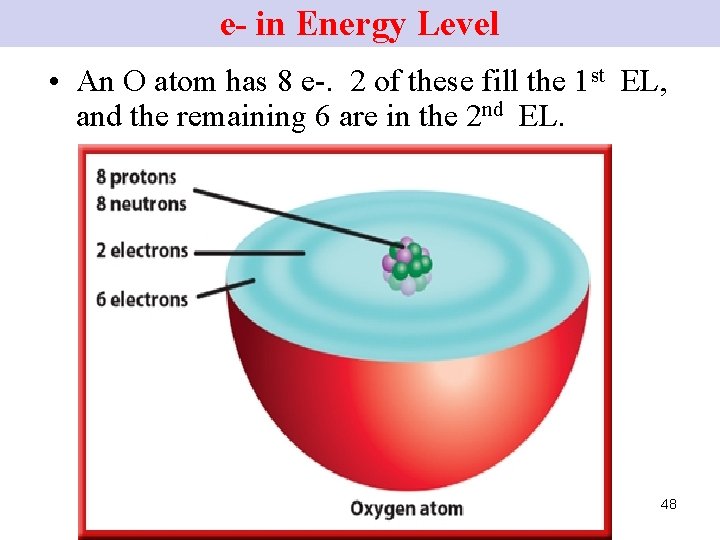
e- in Energy Level • An O atom has 8 e-. 2 of these fill the 1 st EL, and the remaining 6 are in the 2 nd EL. 48

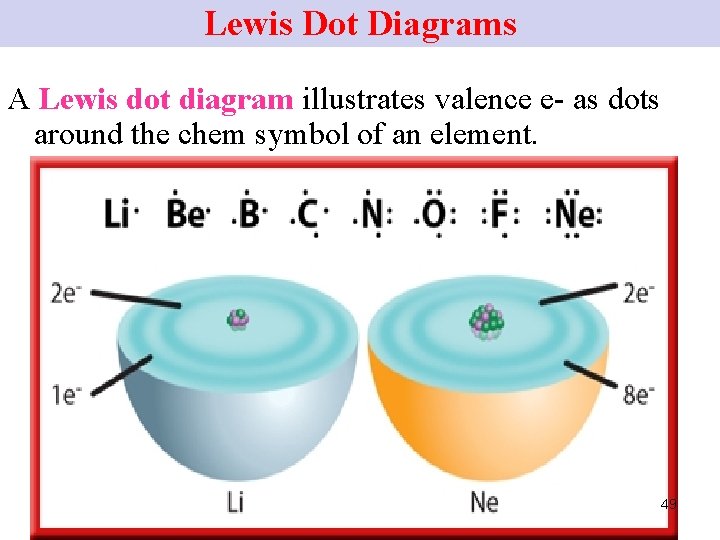
Lewis Dot Diagrams A Lewis dot diagram illustrates valence e- as dots around the chem symbol of an element. 49

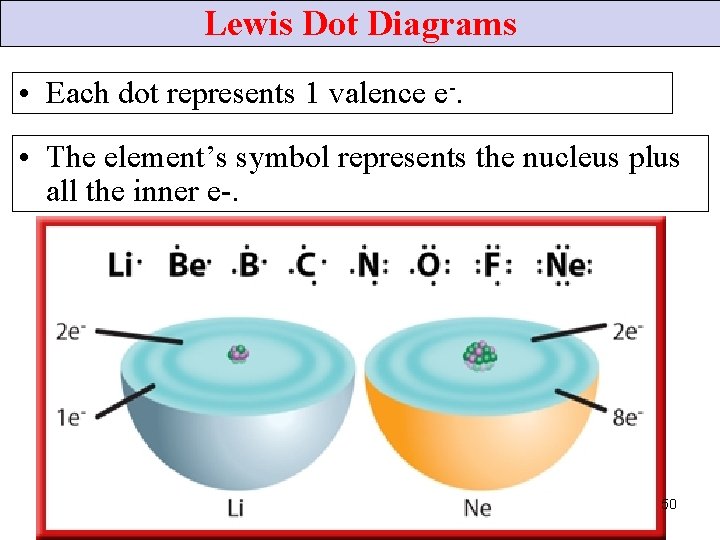
Lewis Dot Diagrams • Each dot represents 1 valence e-. • The element’s symbol represents the nucleus plus all the inner e-. 50

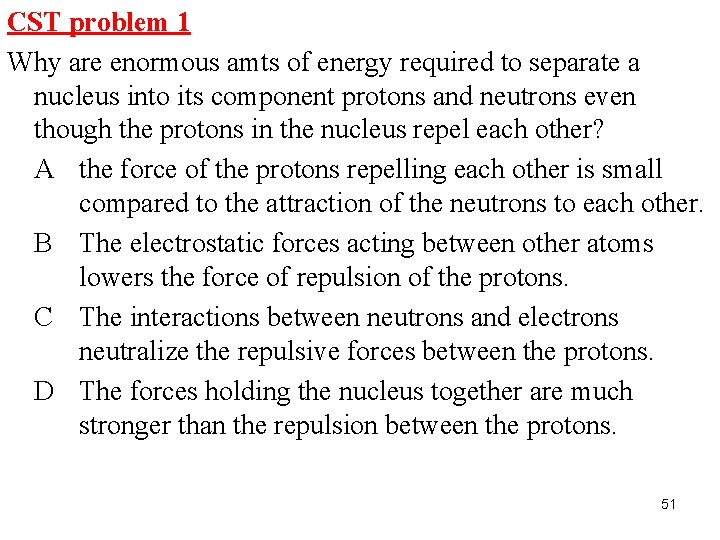
CST problem 1 Why are enormous amts of energy required to separate a nucleus into its component protons and neutrons even though the protons in the nucleus repel each other? A the force of the protons repelling each other is small compared to the attraction of the neutrons to each other. B The electrostatic forces acting between other atoms lowers the force of repulsion of the protons. C The interactions between neutrons and electrons neutralize the repulsive forces between the protons. D The forces holding the nucleus together are much stronger than the repulsion between the protons. 51

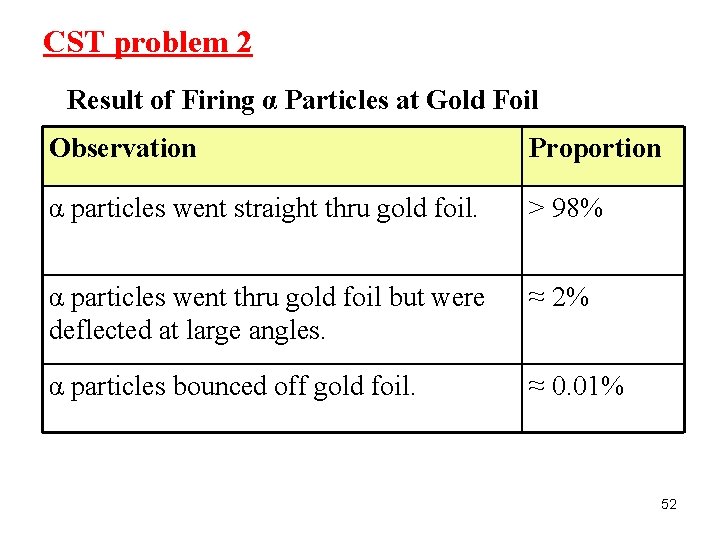
CST problem 2 Result of Firing α Particles at Gold Foil Observation Proportion α particles went straight thru gold foil. > 98% α particles went thru gold foil but were deflected at large angles. ≈ 2% α particles bounced off gold foil. ≈ 0. 01% 52

CST problem 2 (cont. ) What information do the experimental results above reveal about the nucleus of the gold atom? A The nucleus contains less than half the mass of the atom. B The nucleus is small and is the densest part of the atom. C The nucleus contains small positive and negative particles. D The nucleus is large and occupies most of the atom’s space. 53

The End 54