Atomic history Daltons Atomic Theory1700 s 1 2



- Slides: 18

Atomic history

Dalton’s Atomic Theory/1700 s 1. . 2. . 3. . 4. .

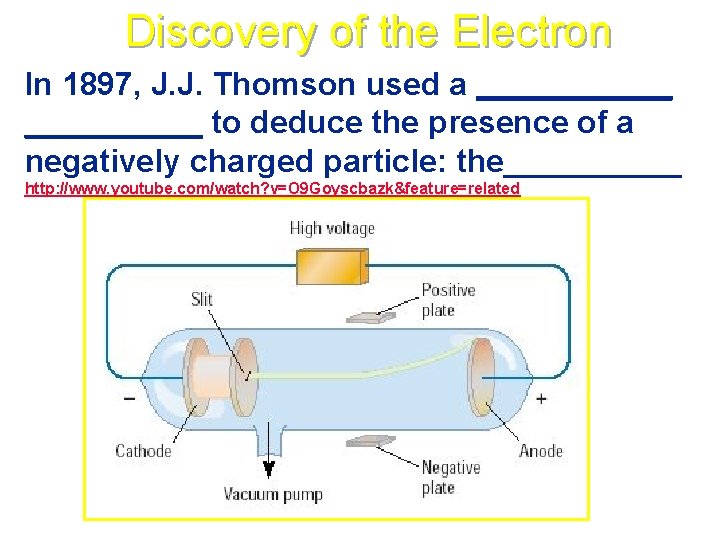
Discovery of the Electron In 1897, J. J. Thomson used a ______ to deduce the presence of a negatively charged particle: the_____ http: //www. youtube. com/watch? v=O 9 Goyscbazk&feature=related

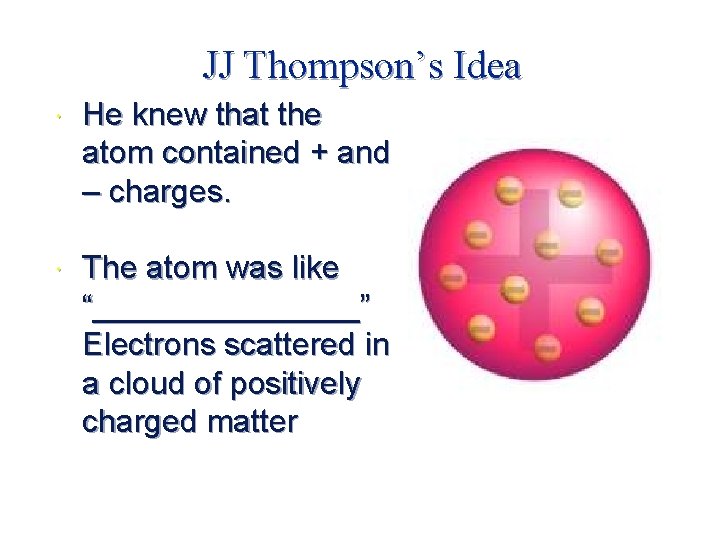
JJ Thompson’s Idea He knew that the atom contained + and – charges. The atom was like “________” Electrons scattered in a cloud of positively charged matter

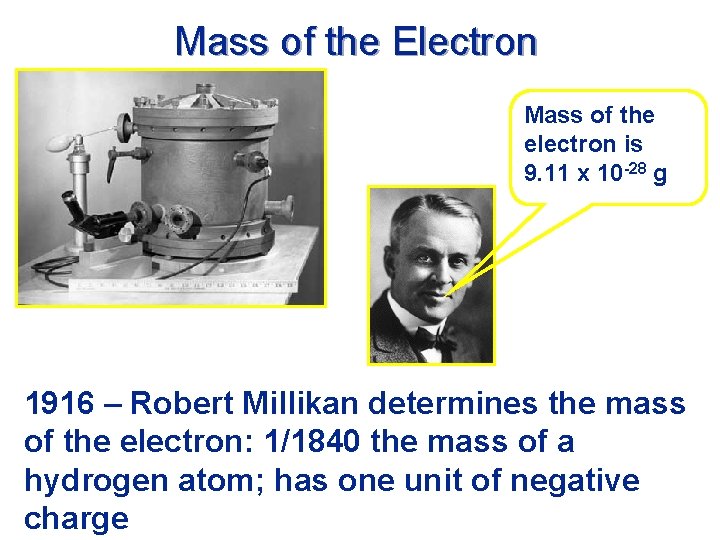
Mass of the Electron Mass of the electron is 9. 11 x 10 -28 g The oil drop apparatus 1916 – Robert Millikan determines the mass of the electron: 1/1840 the mass of a hydrogen atom; has one unit of negative charge

Conclusions from the Study of the Electron: a) Cathode rays have ________ regardless of the element used to produce them. All elements must contain identically charged electrons. b) Atoms are neutral, so there must be ________in the atom to balance the negative charge of the electrons c) _____________that atoms must contain other particles that account for most of the mass

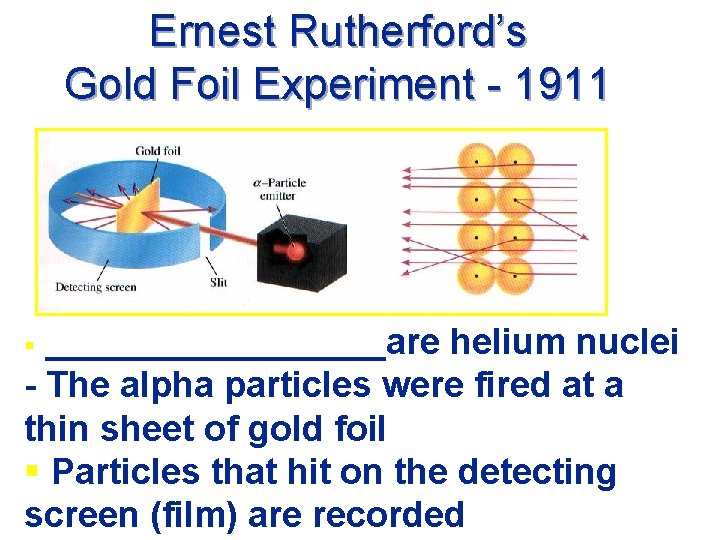
Ernest Rutherford’s Gold Foil Experiment - 1911 _________are helium nuclei - The alpha particles were fired at a thin sheet of gold foil § Particles that hit on the detecting screen (film) are recorded §

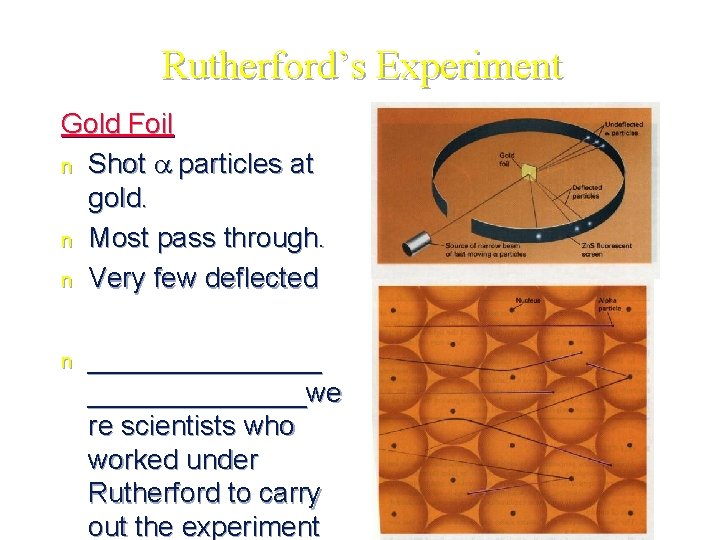
Rutherford’s Experiment Gold Foil n Shot a particles at gold. n Most pass through. n Very few deflected n ________we re scientists who worked under Rutherford to carry out the experiment

Rutherford’s Findings Most of the particles passed right through § A few particles were deflected § VERY FEW were greatly deflected § Conclusions: a) The nucleus is _____ b) The nucleus is _____ c) The nucleus is _____ charged

James Chadwick Studied the mass of the nucleus of the atom and realized there is more mass than number of protons. n The _____ accounted for the extra mass. n It was so hard to find because it is _______ and in the _____. n

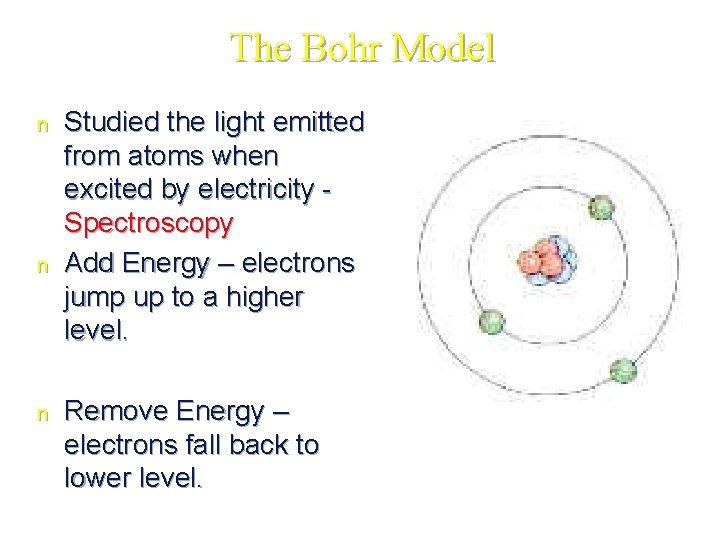
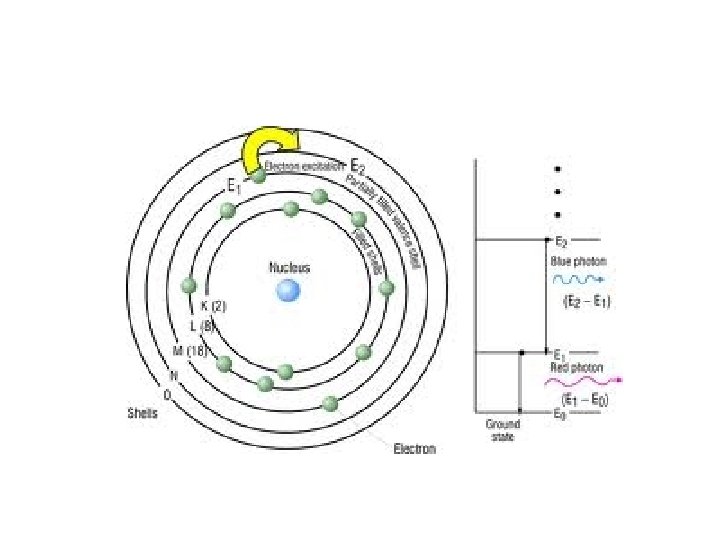
The Bohr Model n n n Studied the light emitted from atoms when excited by electricity Spectroscopy Add Energy – electrons jump up to a higher level. Remove Energy – electrons fall back to lower level.


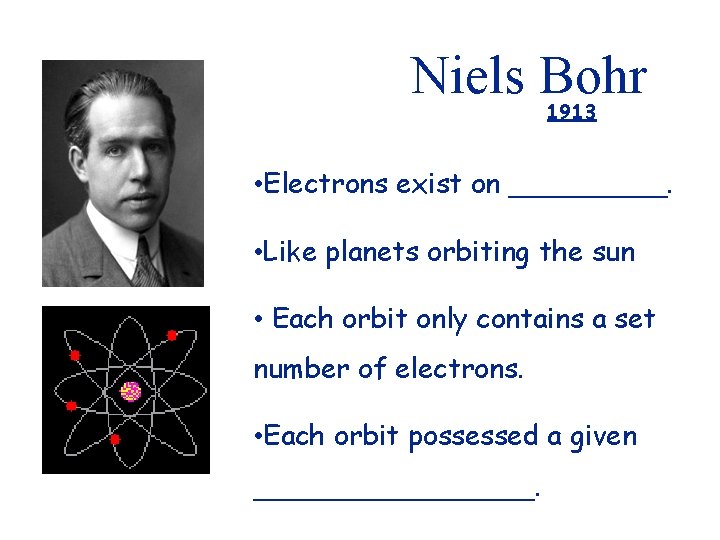
Niels Bohr 1913 • Electrons exist on _____. • Like planets orbiting the sun • Each orbit only contains a set number of electrons. • Each orbit possessed a given ________.

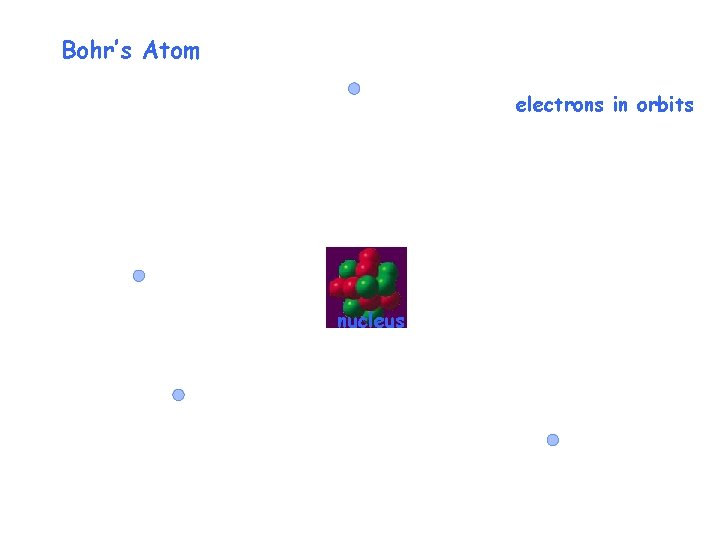
Bohr’s Atom electrons in orbits nucleus

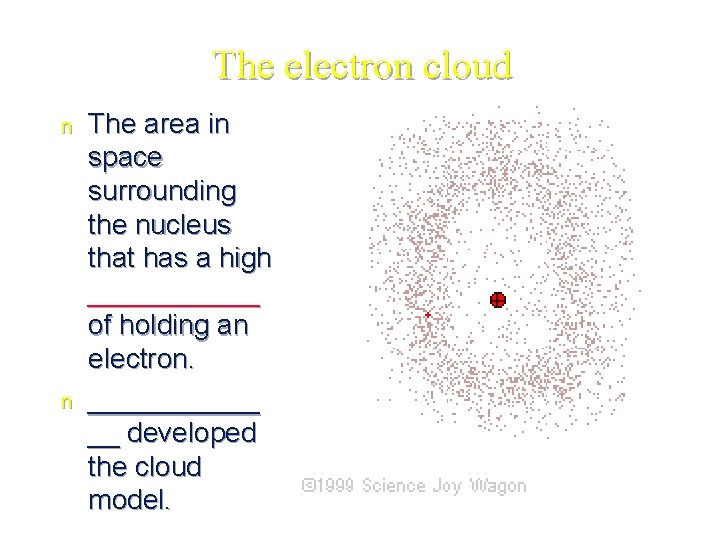
The electron cloud n n The area in space surrounding the nucleus that has a high ______ of holding an electron. ______ __ developed the cloud model.

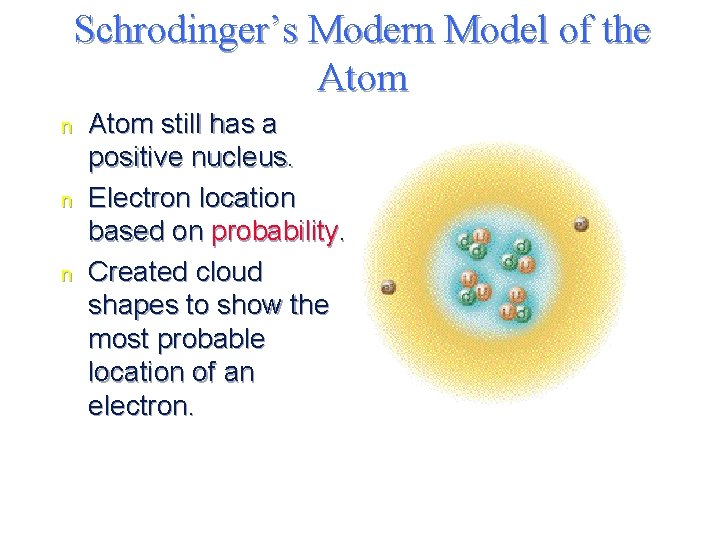
Schrodinger’s Modern Model of the Atom n n n Atom still has a positive nucleus. Electron location based on probability. Created cloud shapes to show the most probable location of an electron.

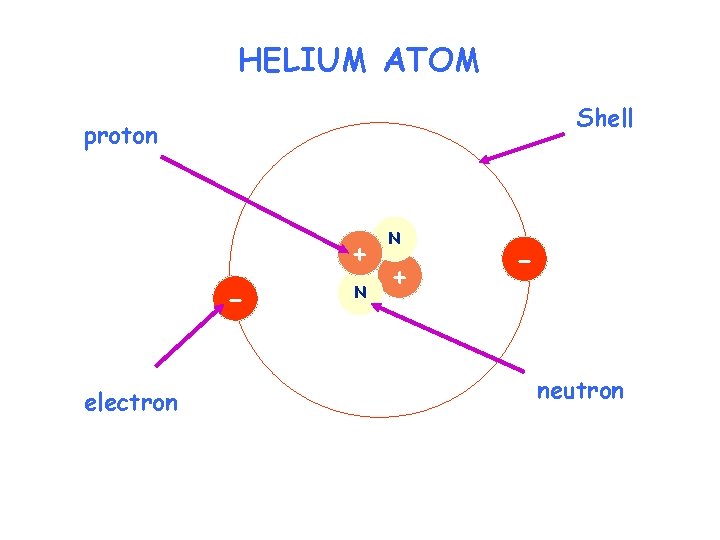
HELIUM ATOM Shell proton + electron N N + - neutron

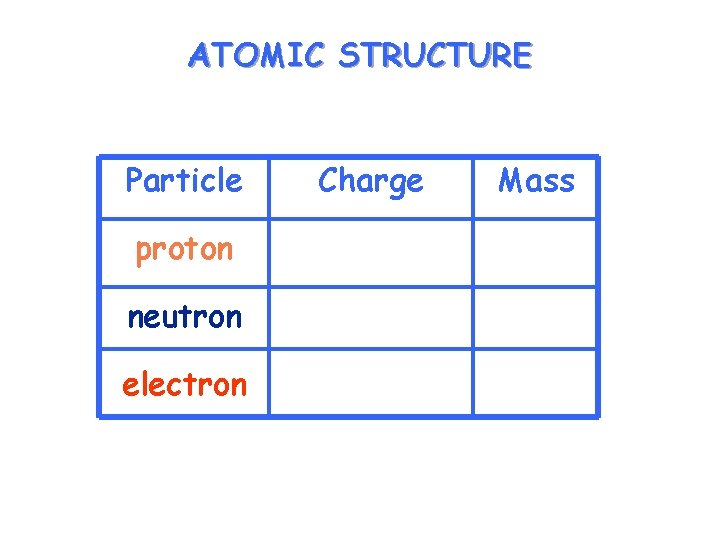
ATOMIC STRUCTURE Particle proton neutron electron Charge Mass