Daltons Atomic Theory Dalton stated that elements consisted





- Slides: 14

Daltons Atomic Theory • Dalton stated that elements consisted of tiny particles called atoms • He also called the elements pure substances because all atoms of an element were identical and that in particular they had the same mass.

Dalton’s Atomic Theory 1. All matter consists of tiny particles. Dalton, like the Greeks, called these particles “atoms”. 2. Atoms of one element can neither be subdivided nor changed into atoms of any other element. 3. Atoms can neither be created nor destroyed. 4. All atoms of the same element are identical in mass, size, and other properties. 5. Atoms of one element differ in mass and other properties from atoms of other elements. 6. In compounds, atoms of different elements combine in simple, whole number ratios.

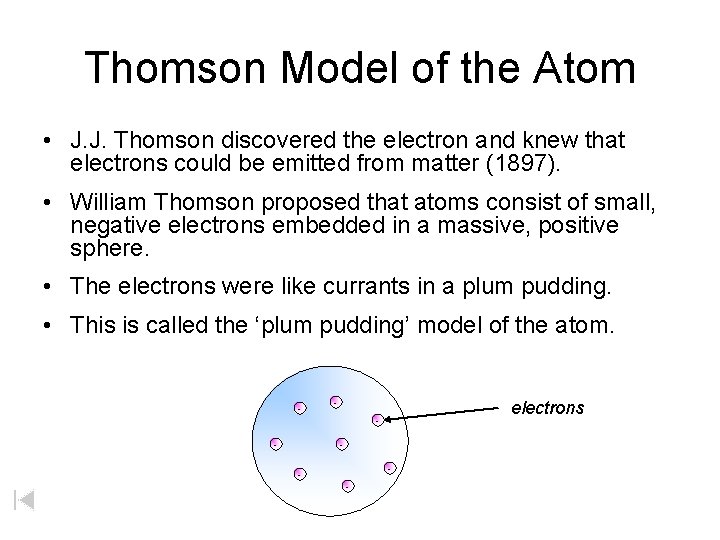
Thomson Model of the Atom • J. J. Thomson discovered the electron and knew that electrons could be emitted from matter (1897). • William Thomson proposed that atoms consist of small, negative electrons embedded in a massive, positive sphere. • The electrons were like currants in a plum pudding. • This is called the ‘plum pudding’ model of the atom. - - electrons - - -

J. J. Thomson • He proved that atoms of any element can be made to emit tiny negative particles. • From this he concluded that ALL atoms must contain these negative particles. • He knew that atoms did not have a net negative charge and so there must be balancing the negative charge. J. J. Thomson

William Thomson (Lord Kelvin) • In 1910 proposed the Plum Pudding model – Negative electrons were embedded into a positively charged spherical cloud. Zumdahl, De. Coste, World of Chemistry 2002, page 56 Spherical cloud of Positive charge Electrons

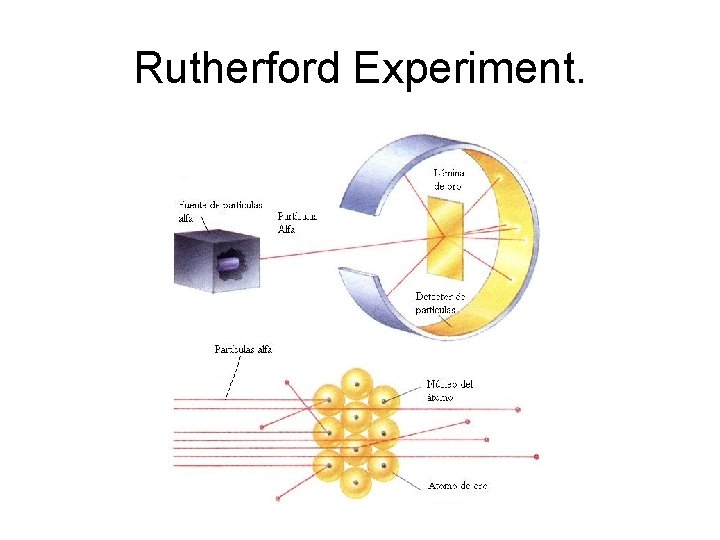
http: • http: //rabfis 15. uco. es/Modelos%20 At%c 3 %b 3 micos%20. NET/Modelos/Experiencias /Experiencia. Rutherford. aspx

Rutherford Experiment.


Structure of the Atom 3: The Rutherford Model • http: //www. youtube. com/watch? v=Ff. Y 4 R 5 mk. MY 8 • http: //www. authorstream. com/Presentation /cchschem-33220 -atomic-structureentertainment-ppt-powerpoint/


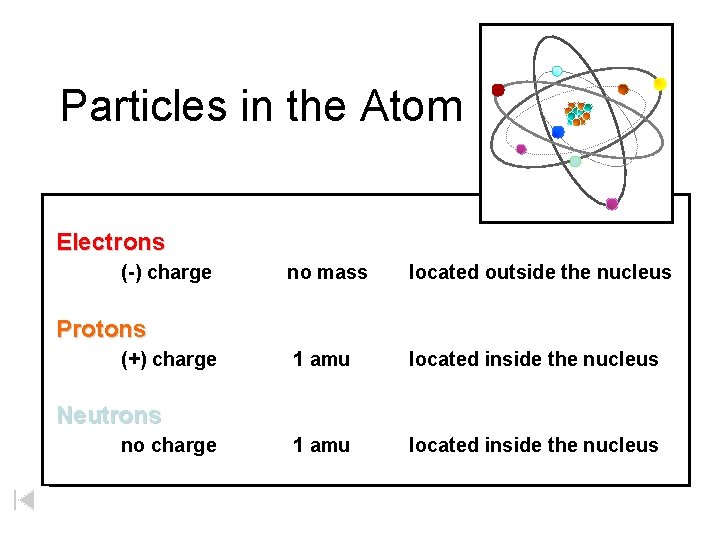
Particles in the Atom Electrons (-) charge no mass located outside the nucleus 1 amu located inside the nucleus Protons (+) charge Neutrons no charge

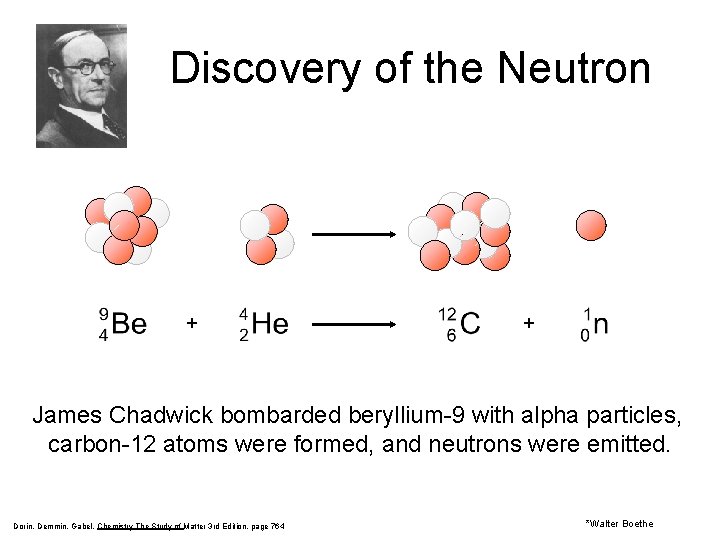
Discovery of the Neutron + + James Chadwick bombarded beryllium-9 with alpha particles, carbon-12 atoms were formed, and neutrons were emitted. Dorin, Demmin, Gabel, Chemistry The Study of Matter 3 rd Edition, page 764 *Walter Boethe

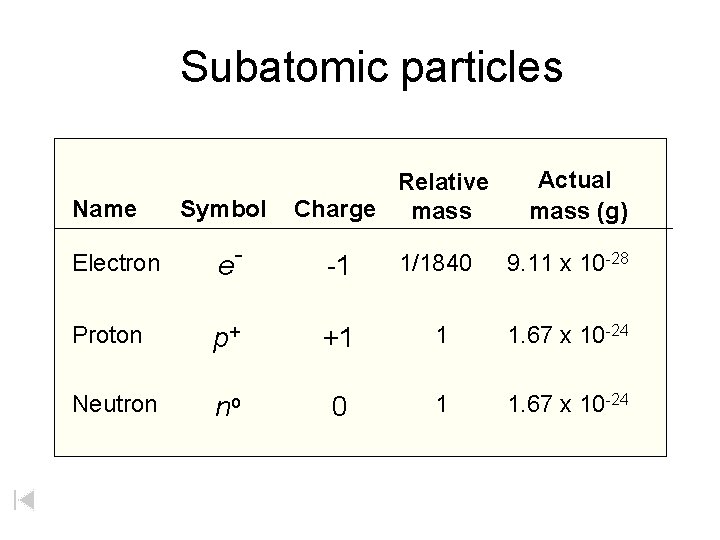
Subatomic particles Name Symbol Relative Charge mass Actual mass (g) Electron e- -1 1/1840 9. 11 x 10 -28 Proton p+ +1 1 1. 67 x 10 -24 Neutron no 0 1 1. 67 x 10 -24

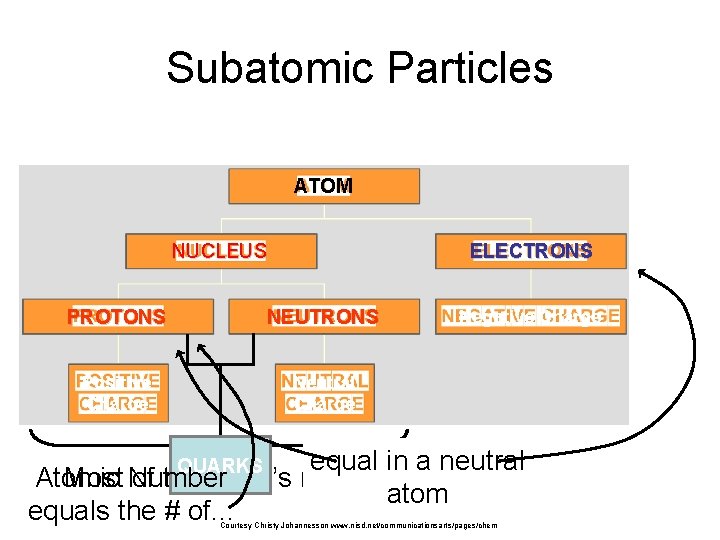
Subatomic Particles ATOM NUCLEUS ELECTRONS PROTONS NEUTRONS Positive Charge Neutral Charge Negative Charge equal in a neutral Atomic Most Number of the atom’s mass. atom equals the # of. . . QUARKS Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem