Atomic Theory 1 Atomic Theory Theories in science















- Slides: 21

Atomic Theory 1

Atomic Theory Theories in science are proposed to explain the evidence available at the time. As new evidence is discovered theories are adapted to explain new data. This is the nature of Science. In the future this will continue.

Plato Democritus 460 -370 BC Atoms make up the world

Plato Teacher of Aristotle 384 -322 BC Four Elements

Forces Aristotle Properties The Four Element Theory wetness harmony conflict Water Wind coldness hotness Earth Fire dryness

The Four Element Theory lasted for about 2 thousand years because no one tested theory with scientific experiments. It was not a scientific theory- which is tested by experiment.

John Dalton 1766 -1844 Atomic Theory

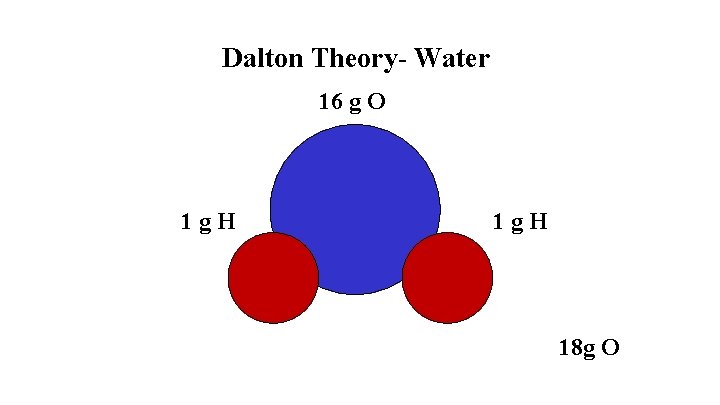
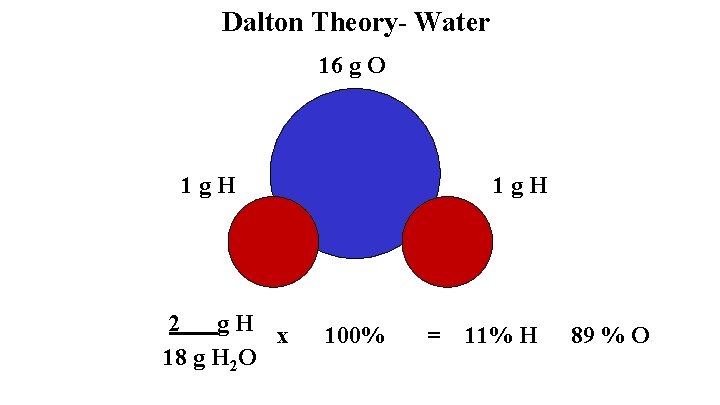
Dalton’s Atomic Theory- 1808 Evidence 1. The Law of Conservation of Mass 2. The Law of Constant Composition- water is 11 % H and 89 % O Theory 1. Each atom is an indestructible and unique spherical particle 2. Atoms combine in simple whole number ratios to form compounds

Hydrogen Oxygen Lithium

Dalton Theory- Water 1 g. H 16 g O 18 g O

Dalton Theory- Water 16 g O 1 g. H 18 g O

Dalton Theory- Water 16 g O 1 g. H 2 g. H x 18 g H 2 O 1 g. H 100% = 11% H 89 % O

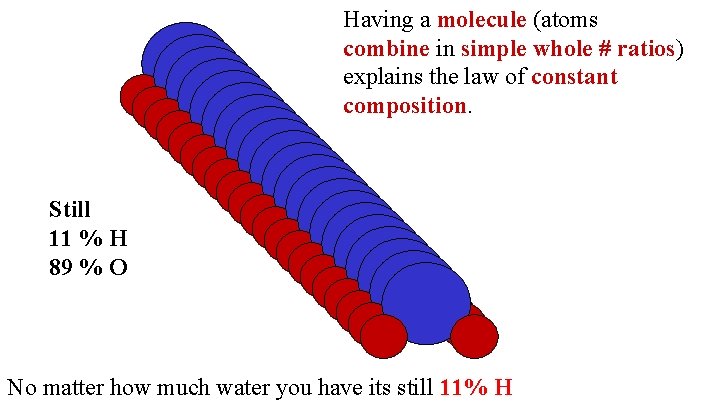
Having a molecule (atoms combine in simple whole # ratios) explains the law of constant composition. Still 11 % H 89 % O No matter how much water you have its still 11% H

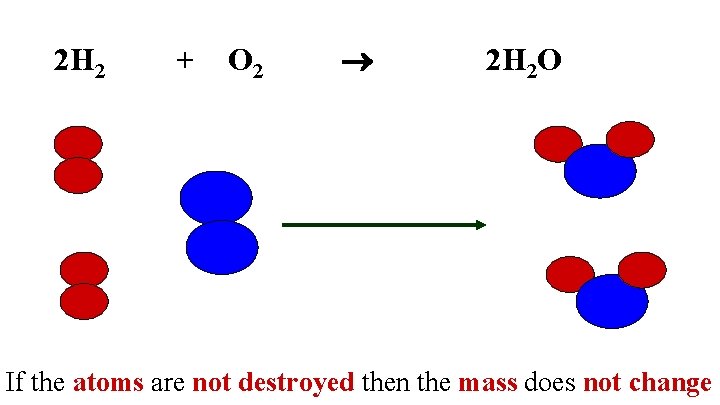
2 H 2 + O 2 2 H 2 O If the atoms are not destroyed then the mass does not change

J. J. Thomson 1871 -1937

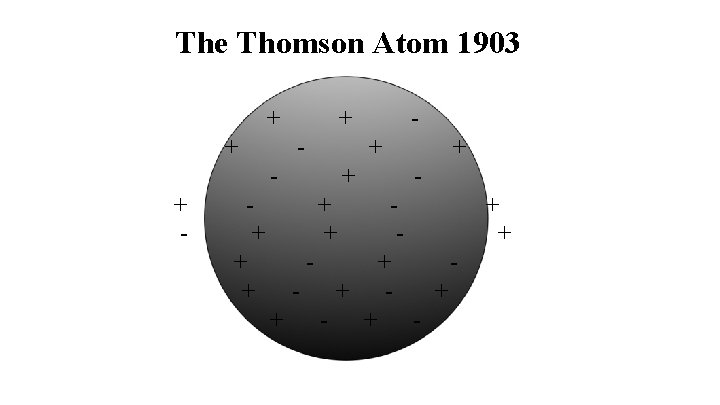
The Thomson Theory of the Atom Evidence 1. The electrical nature of matter beta particles static electricity current electricity Theory 1. The atom is made up of positive material with negative particles throughout- like blueberries in a blueberry muffin.

The Thomson Atom 1903 + + + - + + + -

Ernest Rutherford 1871 -1937

The Rutherford Atom 1911 Simulation Evidence: Gold Foil Experiment 1. 99 % of alphas are not deflected 2. 0. 01 % of alphas are radically deflected Theory 1. Most of the atom is empty space. 2. There is a small dense nucleus in the center of the atom that makes up most of the mass. Electrons circle the nucleus randomly.

The Rutherford Atom 1911 Scale: nucleus is home plate; atom is the baseball field Be Nucleus- is small but has most of the mass Nucleus- 4 protons and 5 neutrons - - - Electrons

Cornelis Escher 1898 -1972 Discovery of the Nucleus Size of the Atom