The Atomic Theory and Electronic Structure A VisualHistorical



































- Slides: 72

The Atomic Theory and Electronic Structure A Visual-Historical Approach David A. Katz Department of Chemistry Pima Community College Tucson, AZ U. S. A. Voice: 520 -206 -6044 Email: dkatz@pima. edu Web site: http: //www. chymist. com

Theories of Matter • The Greeks and Hindus appear to have developed theories on matter. • Most of the writings are attributed to the Greeks due to the amount of recorded information that has survived to the present. • Greeks thought substances could be converted or transformed into other forms. • They observed the changing of states due to heat and equated it with biological processes. • The Greeks were philosophers and thinkers, not experimentalists, so they did not conduct experiments to verify their ideas.

• Thales of Miletus (about 624 -about 527 B. C. ) – Proposed that water is the primal matter from which everything originated. – He is also credited with defining a soul as that which possesses eternal motion. • Anaximander (610 -546 B. C. ) – The primary substance, the apeiron, was eternal and unlimited in extension. It was not composed of any known elements and it possessed eternal motion (i. e. , a soul). • Anaximenes (585 -524 B. C. ) – Stated that air is the primary substance – Suggested it could be transformed into other substances by thinning (fire) or thickening (wind, clouds, rain, hail, earth, rock).

• Heraclitus of Ephesus (544 -484 B. C. ) – fire is the primeval substance – Change is the only reality. • The Pythagoreans (Pythagoras (570 -490 B. C. )) – Reduced theory of matter to a mathematical and geometric basis by using geometric solids to represent the basic elements: • • • cube = earth octahedron = air tetrahedron = fire icosahedron = water dodecahedron = ether • Empedocles of Agrigentum (492 -432 B. C. ) – Credited with the first announcement of the concept of four elements: earth, air, fire, and water, which were capable of combining to form all other substances. – Elements combined by specific attractions or repulsions which were typified as love and hate.

• Anaxagoras of Klazomenae (c. 500 -428 B. C. ) – Considered the universe to be composed of an infinite variety of small particles called seeds. – These seeds were infinitely divisible and possessed a quality which allowed "like to attract like" to form substances such a flesh, bone, gold, etc. • Leucippus (5 th century B. C. ) and Democritus (460370 B. C. ) – First atomic theory. – All material things consisted of small indivisible particles, or atoms, which were all qualitatively alike, differing only in size, shape, position and mass. – Atoms, they stated, exist in a vacuous space which separates them and, because of this space, they are capable of movement. (This can be considered at the first kinetic theory. )

• Pierre Gassendi (1592 -1655) – Revived the atomic theory (1650) • Atoms are primordial, impenetable, simple, unchangeable, and indestructible bodies • They are the smallest bodies that can exist • Atoms and vacuum, the absolutely full and the absolutely empty, are the only true principles and there is no third principle possible. • Atoms differ in size, shape and weight • Atoms may possess hooks and other excrescences • Atoms possess motion • Atoms form very small corpuscles, or molecules, which aggregate into larger and larger bodies

• Robert Boyle (1627 -1691) – Hypothesized a universal matter, the concept of atoms of different shapes and sizes – Defined an element (The Sceptical Chymist, 1661) • And, to prevent mistakes, I must advertise You, that I now mean by Elements, as those Chymists that speak plainest do by their Principles, certain Primitive and Simple, or perfectly unmingled bodies; which not being made of any other bodies, or of one another, are the Ingredients of which all those call’d perfectly mixt Bodies are immediately compounded, and into which they are ultimately resolved. – He could not give any examples of elements that fit his definition.

• Sir Isaac Newton (1642 -1727) – Modified atomic theory to atoms as hard particles with forces of attraction between them

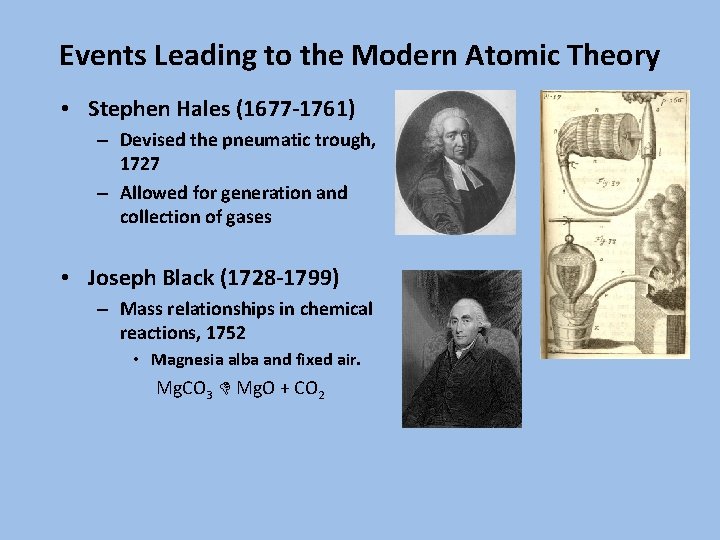
Events Leading to the Modern Atomic Theory • Stephen Hales (1677 -1761) – Devised the pneumatic trough, 1727 – Allowed for generation and collection of gases • Joseph Black (1728 -1799) – Mass relationships in chemical reactions, 1752 • Magnesia alba and fixed air. Mg. CO 3 Mg. O + CO 2

• Henry Cavendish (1731 -1810) – Inflammable air, “Hydrogen”, 1766 – Later: H 2 + O 2 → H 2 O • Joseph Priestley (1733 -1804) and Carl Wilhelm Scheele (1742 -1786) – Dephlogisticated air/ feuer luft “Oxygen”, 1774

• Antoine Laurent Lavoisier (1743 -1794) (and Marie. Anne Pierrette Paulze Lavoisier (1758 -1836)? ) – Nature of combustion, 1777 – Elements in Traité élémentaire de chemie, 1789

The Atomic Theory • John Dalton (1766 -1844) – New System of Chemical Philosophy, 1808 – All bodies are constituted of a vast number of extremely small particles, or atoms of matter bound together by a force of attraction – The ultimate particles of all homogeneous bodies are perfectly alike in weight, figure, etc.

The Atomic Theory – Atoms have definite relative weights “expressed in atoms of hydrogen, each of which is denoted by unity” – Atoms combine in simple numerical ratios to form compounds – Under given experimental conditions a particular atom will always behave in the same manner – Atoms are indestructible


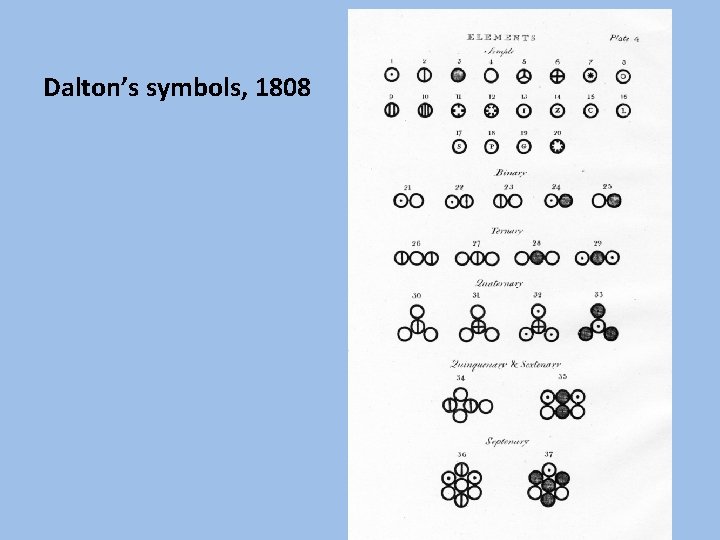
Dalton’s symbols, 1808

Dalton’s atomic weights, 1808

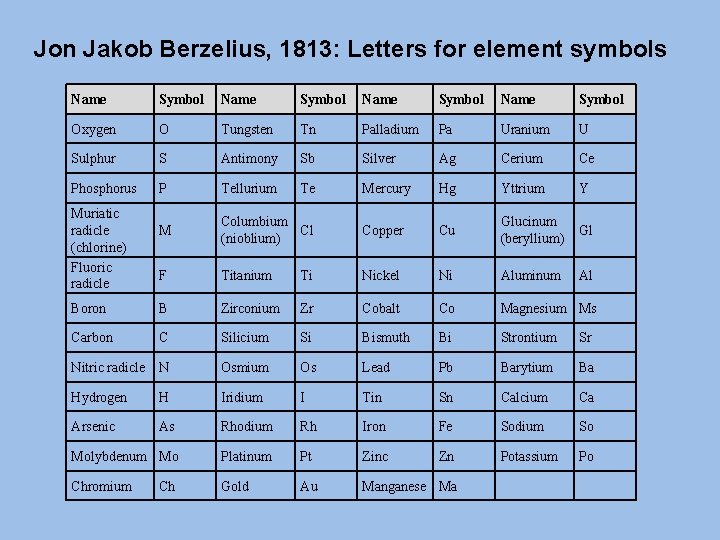
Jon Jakob Berzelius, 1813: Letters for element symbols Name Symbol Oxygen O Tungsten Tn Palladium Pa Uranium U Sulphur S Antimony Sb Silver Ag Cerium Ce Phosphorus P Tellurium Te Mercury Hg Yttrium Y M Columbium Cl (nioblium) Copper Cu Glucinum (beryllium) Gl F Titanium Ti Nickel Ni Aluminum Al Boron B Zirconium Zr Cobalt Co Magnesium Ms Carbon C Silicium Si Bismuth Bi Strontium Sr Nitric radicle N Osmium Os Lead Pb Barytium Ba Hydrogen H Iridium I Tin Sn Calcium Ca Arsenic As Rhodium Rh Iron Fe Sodium So Molybdenum Mo Platinum Pt Zinc Zn Potassium Po Chromium Gold Au Manganese Ma Muriatic radicle (chlorine) Fluoric radicle Ch

Pieces of Atoms – the electron • Heinrich Geissler (1814 -1879) • Julius Plücker (1801 -1868) – Evacuated tube glowed, 1859 – Rays affected by a magnet

• Johann Wilhelm Hittorf (1824 -1914) – Maltese cross tube, 1869 • Rays travel in straight line • Cast shadows of objects

• William Crookes (1832 -1919) – Verified previous observations, 1879 – Caused pinwheel to turn • Composed of particles – Have negative charge

• Joseph John Thomson (1846 -1940) e/m = -1. 759 x 108 coulomb/gram - 1897

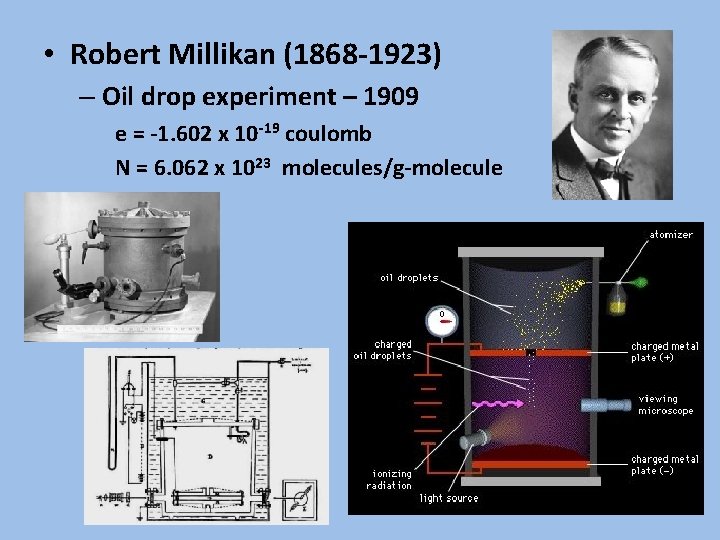
• Robert Millikan (1868 -1923) – Oil drop experiment – 1909 e = -1. 602 x 10 -19 coulomb N = 6. 062 x 1023 molecules/g-molecule

Pieces of Atoms – the proton • Eugen Goldstein (1850 -1930) – Canal rays - 1886

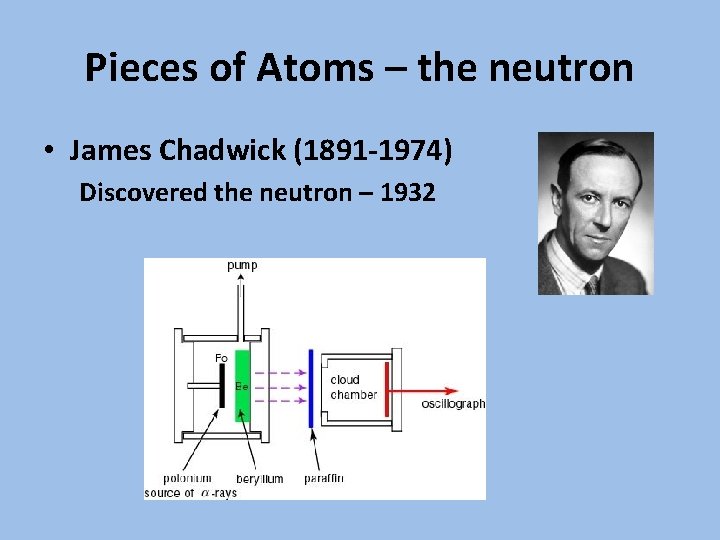
Pieces of Atoms – the neutron • James Chadwick (1891 -1974) Discovered the neutron – 1932

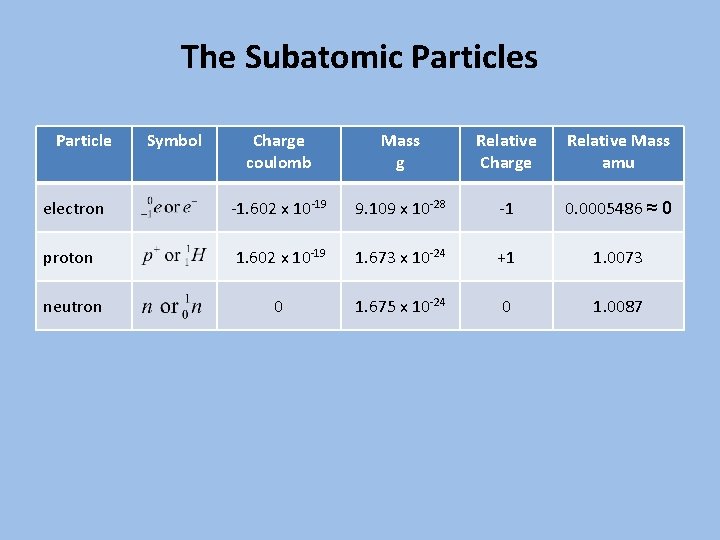
The Subatomic Particles Particle Symbol Charge coulomb Mass g Relative Charge Relative Mass amu electron -1. 602 x 10 -19 9. 109 x 10 -28 -1 0. 0005486 ≈ 0 proton 1. 602 x 10 -19 1. 673 x 10 -24 +1 1. 0073 neutron 0 1. 675 x 10 -24 0 1. 0087

Models of the Atom • Philipp Lenard (1862 -1947) – Dynamids – 1903 • Hantaro Nagaoka (1865 -1950) – Saturnian model - 1904

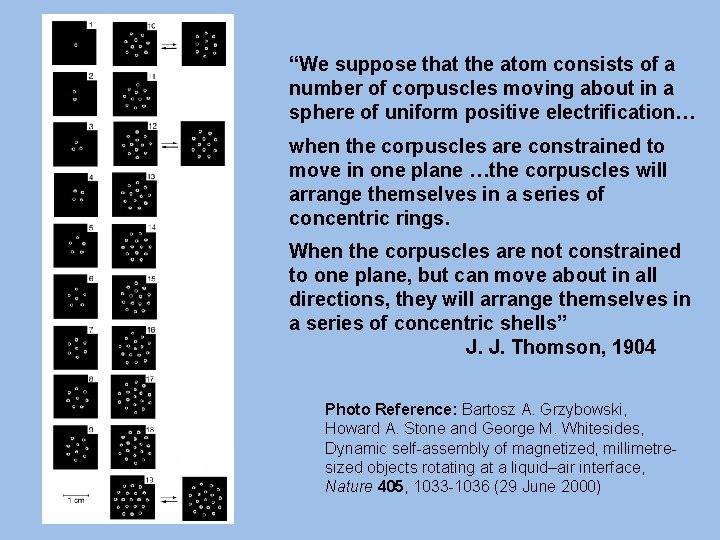
• J. J. Thomson – Plum pudding – 1904 • Partly based on A. M. Mayer’s (1836 -1897) floating magnet experiment A. M. Mayer

“We suppose that the atom consists of a number of corpuscles moving about in a sphere of uniform positive electrification… when the corpuscles are constrained to move in one plane …the corpuscles will arrange themselves in a series of concentric rings. When the corpuscles are not constrained to one plane, but can move about in all directions, they will arrange themselves in a series of concentric shells” J. J. Thomson, 1904 Photo Reference: Bartosz A. Grzybowski, Howard A. Stone and George M. Whitesides, Dynamic self-assembly of magnetized, millimetresized objects rotating at a liquid–air interface, Nature 405, 1033 -1036 (29 June 2000)

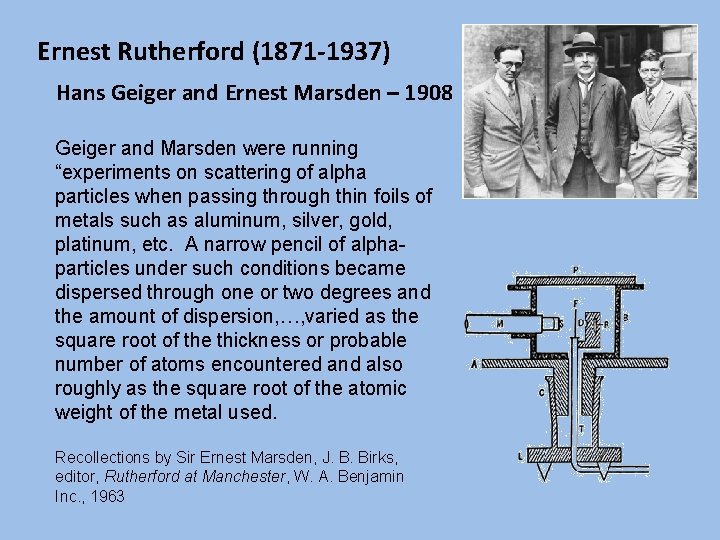
Ernest Rutherford (1871 -1937) Hans Geiger and Ernest Marsden – 1908 Geiger and Marsden were running “experiments on scattering of alpha particles when passing through thin foils of metals such as aluminum, silver, gold, platinum, etc. A narrow pencil of alphaparticles under such conditions became dispersed through one or two degrees and the amount of dispersion, …, varied as the square root of the thickness or probable number of atoms encountered and also roughly as the square root of the atomic weight of the metal used. Recollections by Sir Ernest Marsden, J. B. Birks, editor, Rutherford at Manchester, W. A. Benjamin Inc. , 1963

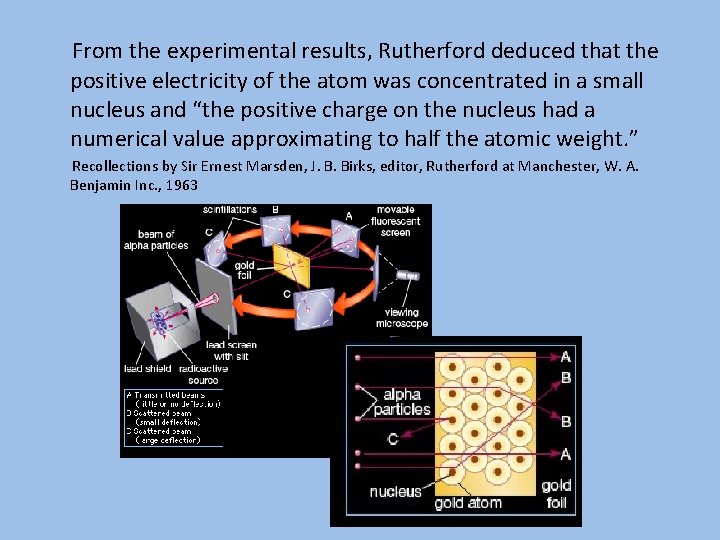
In a discussion with Geiger, regarding Ernest Marsden, Rutherford stated that “I agreed with Geiger that young Marsden, whom he had been training in radioactive methods, ought to begin a research. Why not let him see if any αparticles can be scattered through a large angle? I did not believe they would be…” Recollections by Ernest Rutherford, J. B. Birks, editor, Rutherford at Manchester, W. A. Benjamin Inc. , 1963 “The observations, however, of Geiger and Marsden** on the scattering of a rays indicate that some of the α particles, about 1 in 20, 000 were turned through an average angle of 90 degrees in passing though a layer of gold-foil about 0. 00004 cm. thick, … It seems reasonable to suppose that the deflexion through a large angle is due to a single atomic encounter, …” ** Proc. Roy. Soc. lxxxii, p. 495 (1909) *** Proc. Roy. Soc. lxxxiii, p. 492 (1910)

From the experimental results, Rutherford deduced that the positive electricity of the atom was concentrated in a small nucleus and “the positive charge on the nucleus had a numerical value approximating to half the atomic weight. ” Recollections by Sir Ernest Marsden, J. B. Birks, editor, Rutherford at Manchester, W. A. Benjamin Inc. , 1963

“It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you had fired a 15 -inch shell at a piece of tissue-paper and it came back and hit you. ” Recollections by Ernest Rutherford, J. B. Birks, editor, Rutherford at Manchester, W. A. Benjamin Inc. , 1963

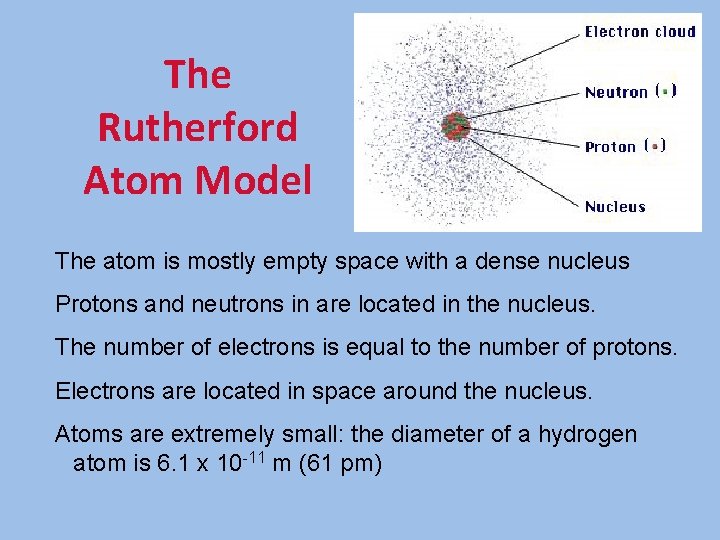
The Rutherford Atom Model The atom is mostly empty space with a dense nucleus Protons and neutrons in are located in the nucleus. The number of electrons is equal to the number of protons. Electrons are located in space around the nucleus. Atoms are extremely small: the diameter of a hydrogen atom is 6. 1 x 10 -11 m (61 pm)

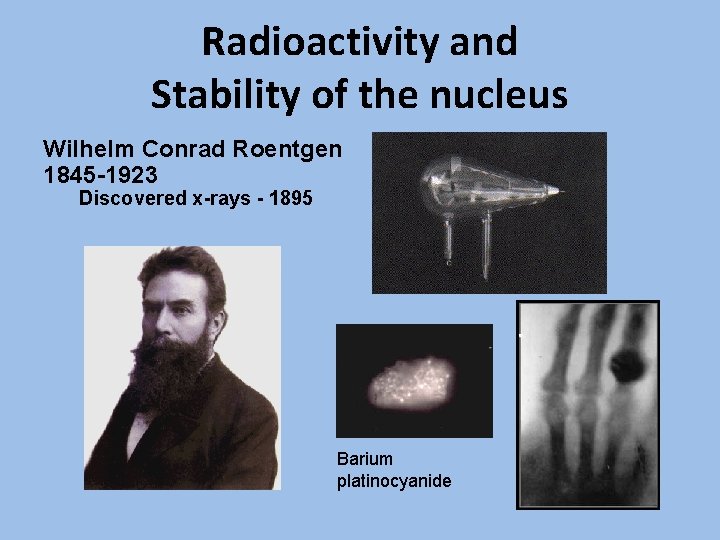
Radioactivity and Stability of the nucleus Wilhelm Conrad Roentgen 1845 -1923 Discovered x-rays - 1895 Barium platinocyanide

Henri Becquerel (1852 -1908) Radiation activity, 1896 Uranium nitrate Image of potassium uranyl sulfate

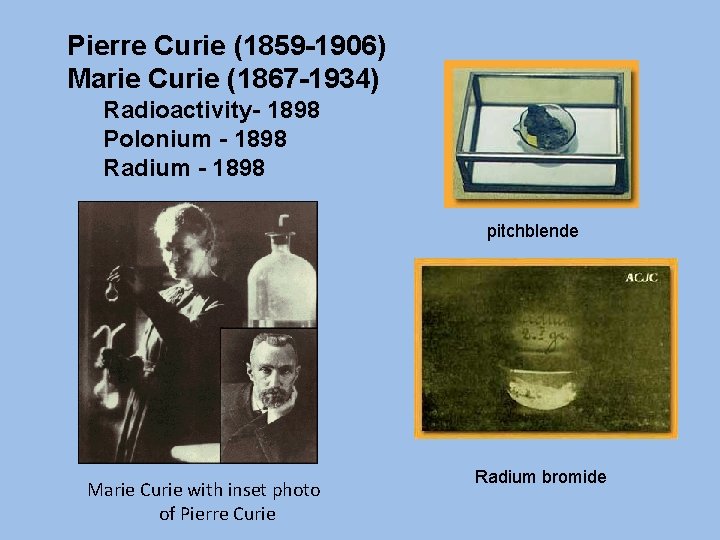
Pierre Curie (1859 -1906) Marie Curie (1867 -1934) Radioactivity- 1898 Polonium - 1898 Radium - 1898 pitchblende Marie Curie with inset photo of Pierre Curie Radium bromide

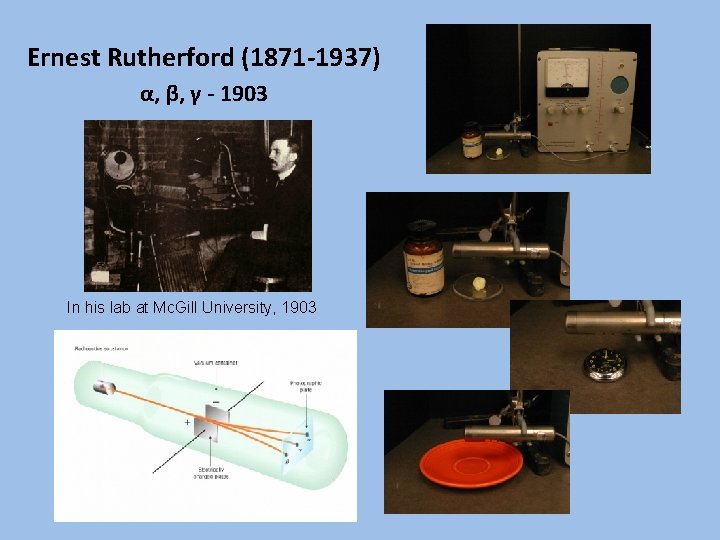
Ernest Rutherford (1871 -1937) α, β, γ - 1903 In his lab at Mc. Gill University, 1903

Glenn T. Seaborg (1912 -1999) Extending the periodic table

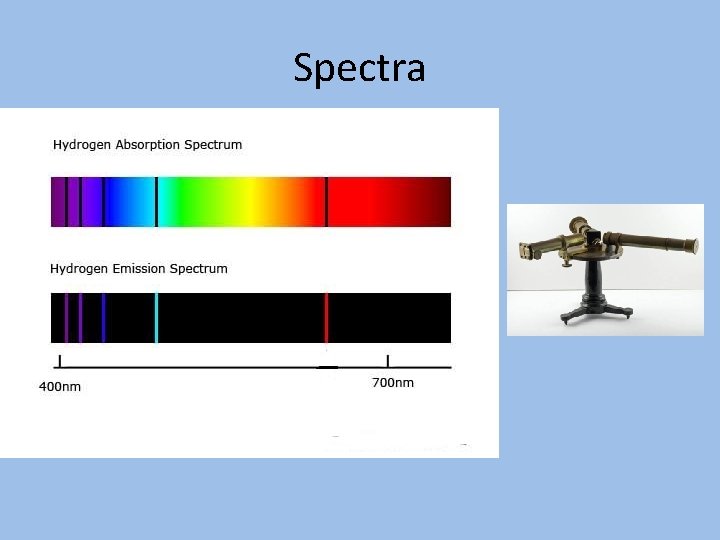
Spectra

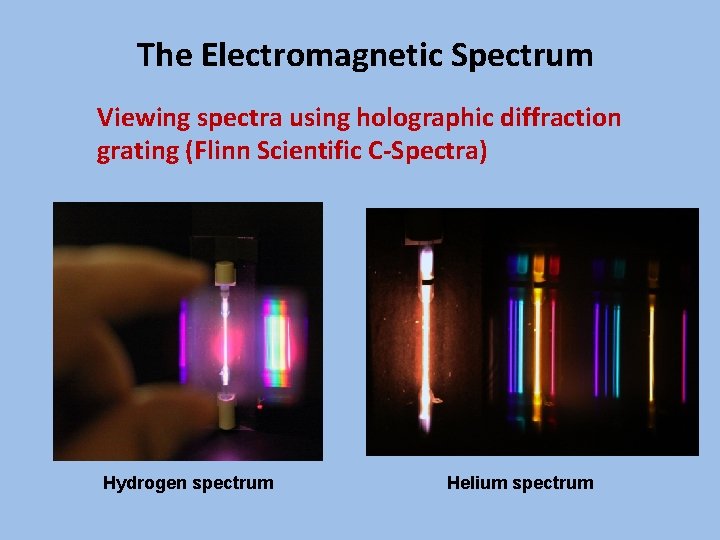
The Electromagnetic Spectrum Viewing spectra using holographic diffraction grating (Flinn Scientific C-Spectra) Hydrogen spectrum Helium spectrum

The Balmer Series of Hydrogen Lines • In 1885, Johann Jakob Balmer (1825 - 1898), worked out a formula to calculate the positions of the spectral lines of the visible hydrogen spectrum Where m = an integer, 3, 4, 5, … • In 1888, Johannes Rydberg generalized Balmer’s formula to calculate all the lines of the hydrogen spectrum Where RH = 109677. 58 cm-1

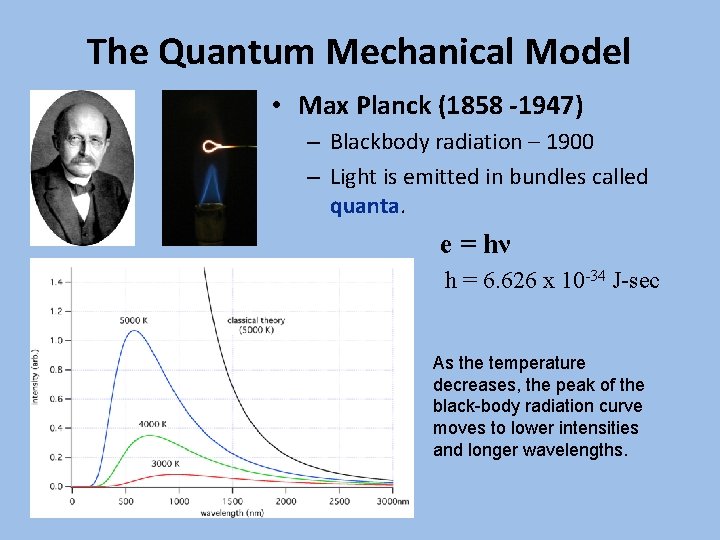
The Quantum Mechanical Model • Max Planck (1858 -1947) – Blackbody radiation – 1900 – Light is emitted in bundles called quanta. e = hν h = 6. 626 x 10 -34 J-sec As the temperature decreases, the peak of the black-body radiation curve moves to lower intensities and longer wavelengths.

The Quantum Mechanical Model • Albert Einstein (1879 -1955) The photoelectric effect – 1905 Planck’s equation: e = hν Equation for light : c = λν Rearrange to Substitute into Planck’s equation From general relativity: e = mc 2 Substitute for e and solve for λ Light is composed of particles called photons

The Bohr Model - 1913 • Niels Bohr (1885 -1962)

The Bohr Model – Bohr’s Postulates 1. Spectral lines are produced by atoms one at a time 2. A single electron is responsible for each line 3. The Rutherford nuclear atom is the correct model 4. The quantum laws apply to jumps between different states characterized by discrete values of angular momentum and energy

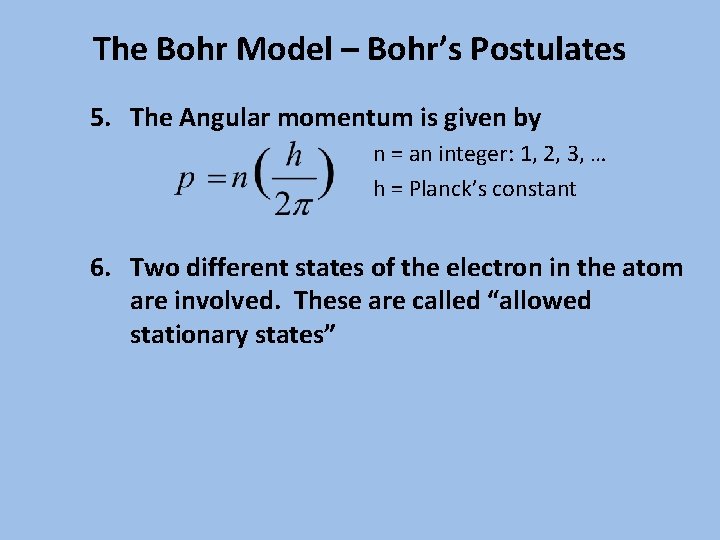
The Bohr Model – Bohr’s Postulates 5. The Angular momentum is given by n = an integer: 1, 2, 3, … h = Planck’s constant 6. Two different states of the electron in the atom are involved. These are called “allowed stationary states”

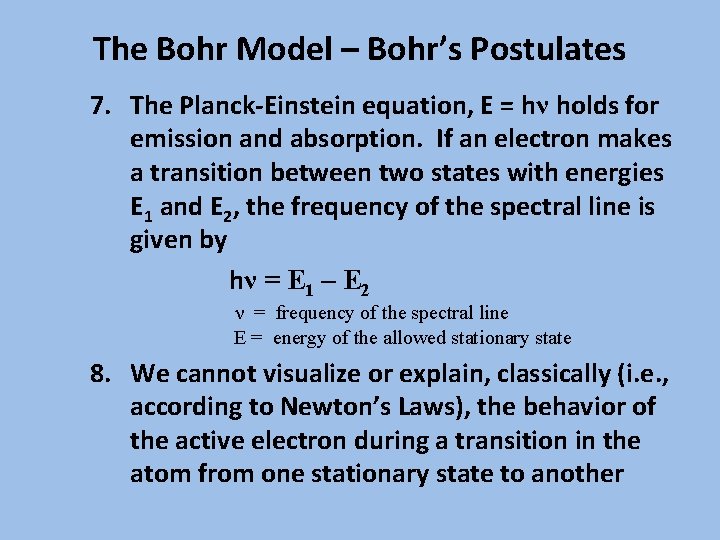
The Bohr Model – Bohr’s Postulates 7. The Planck-Einstein equation, E = hν holds for emission and absorption. If an electron makes a transition between two states with energies E 1 and E 2, the frequency of the spectral line is given by hν = E 1 – E 2 ν = frequency of the spectral line E = energy of the allowed stationary state 8. We cannot visualize or explain, classically (i. e. , according to Newton’s Laws), the behavior of the active electron during a transition in the atom from one stationary state to another

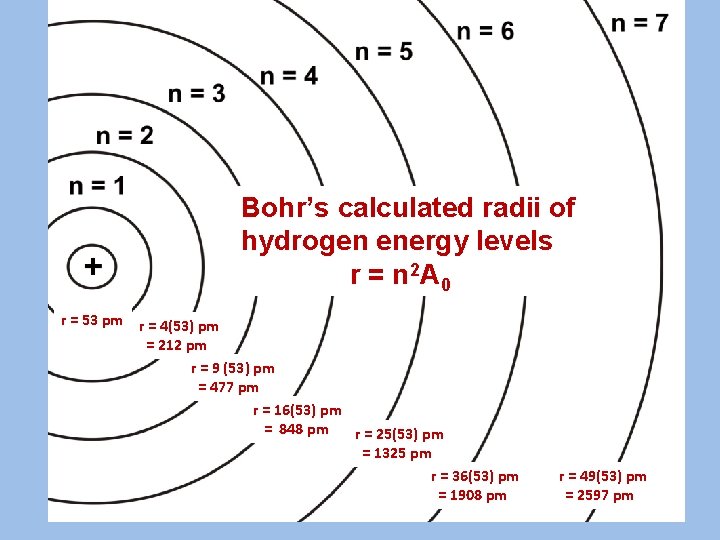
Bohr’s calculated radii of hydrogen energy levels r = n 2 A 0 r = 53 pm r = 4(53) pm = 212 pm r = 9 (53) pm = 477 pm r = 16(53) pm = 848 pm r = 25(53) pm = 1325 pm r = 36(53) pm r = 49(53) pm = 1908 pm = 2597 pm

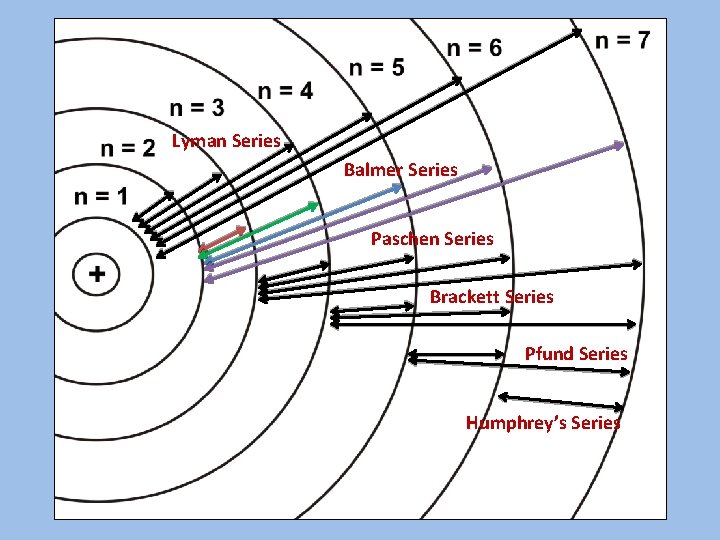
Lyman Series Balmer Series Paschen Series Brackett Series Pfund Series Humphrey’s Series

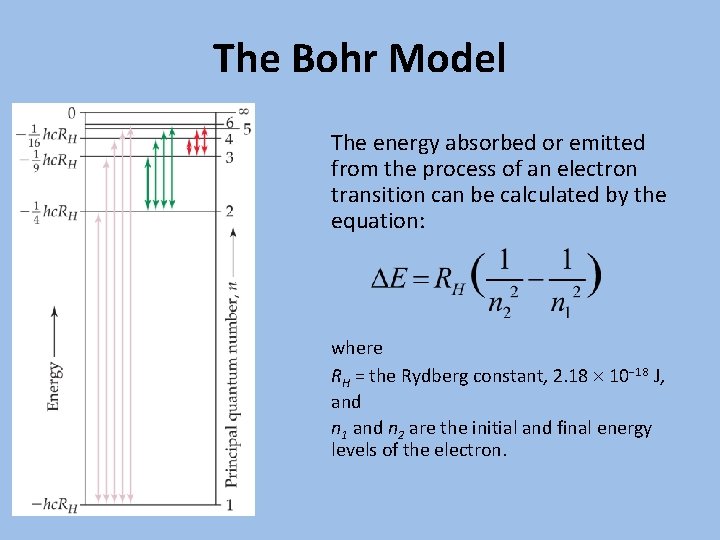
The Bohr Model The energy absorbed or emitted from the process of an electron transition can be calculated by the equation: where RH = the Rydberg constant, 2. 18 10− 18 J, and n 1 and n 2 are the initial and final energy levels of the electron.

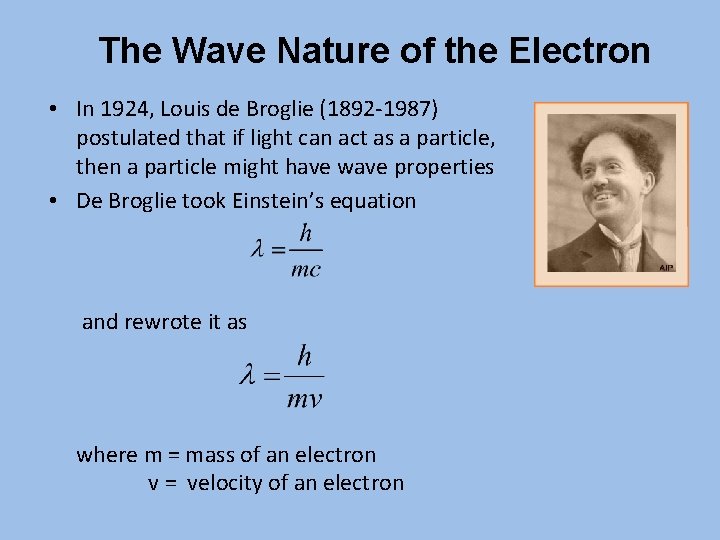
The Wave Nature of the Electron • In 1924, Louis de Broglie (1892 -1987) postulated that if light can act as a particle, then a particle might have wave properties • De Broglie took Einstein’s equation and rewrote it as where m = mass of an electron v = velocity of an electron

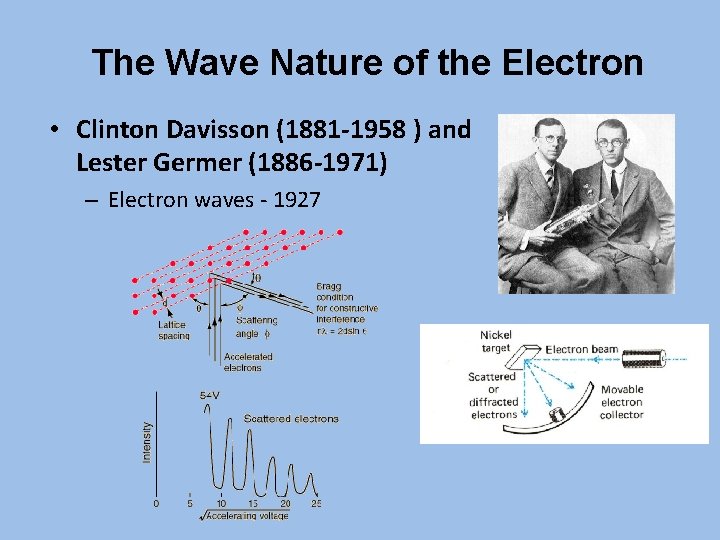
The Wave Nature of the Electron • Clinton Davisson (1881 -1958 ) and Lester Germer (1886 -1971) – Electron waves - 1927

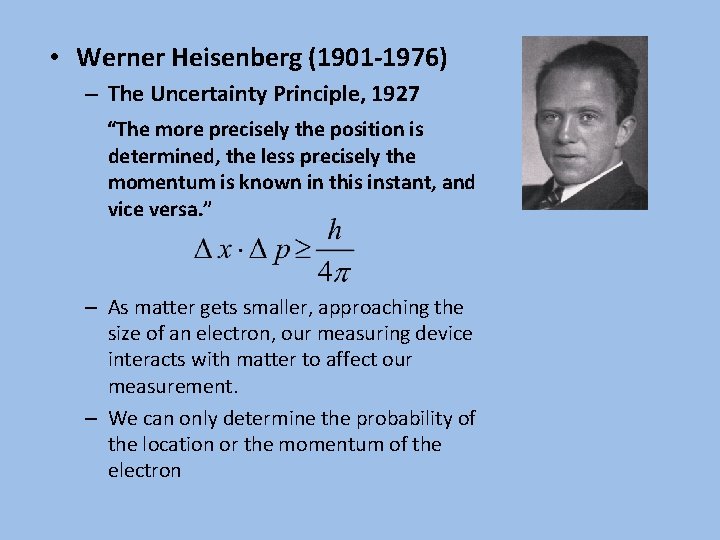
• Werner Heisenberg (1901 -1976) – The Uncertainty Principle, 1927 “The more precisely the position is determined, the less precisely the momentum is known in this instant, and vice versa. ” – As matter gets smaller, approaching the size of an electron, our measuring device interacts with matter to affect our measurement. – We can only determine the probability of the location or the momentum of the electron

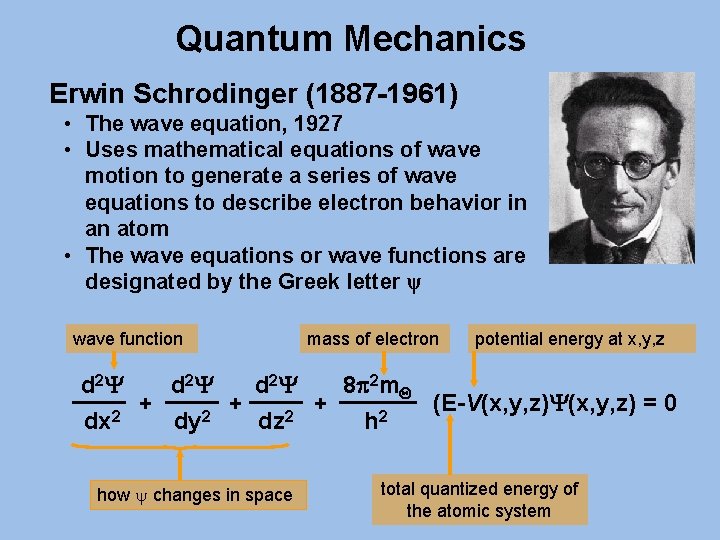
Quantum Mechanics Erwin Schrodinger (1887 -1961) • The wave equation, 1927 • Uses mathematical equations of wave motion to generate a series of wave equations to describe electron behavior in an atom • The wave equations or wave functions are designated by the Greek letter ψ wave function mass of electron potential energy at x, y, z d 2 Y 8 p 2 m. Q + + + (E-V(x, y, z)Y(x, y, z) = 0 2 2 dx dy dz h how y changes in space total quantized energy of the atomic system

Quantum Mechanics • The square of the wave equation, ψ2, gives a probability density map of where an electron has a certain statistical likelihood of being at any given instant in time.

Quantum Numbers • Solving the wave equation gives a set of wave functions, or orbitals, and their corresponding energies. • Each orbital describes a spatial distribution of electron density. • An orbital is described by a set of three quantum numbers. • Quantum numbers can be considered to be “coordinates” (similar to x, y, and z coodrinates for a graph) which are related to where an electron will be found in an atom.

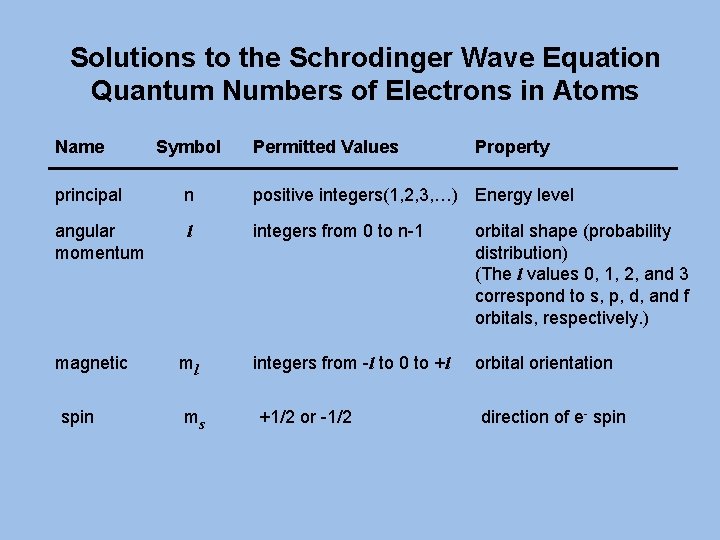
Solutions to the Schrodinger Wave Equation Quantum Numbers of Electrons in Atoms Name Symbol Permitted Values Property principal n positive integers(1, 2, 3, …) Energy level angular momentum l integers from 0 to n-1 orbital shape (probability distribution) (The l values 0, 1, 2, and 3 correspond to s, p, d, and f orbitals, respectively. ) integers from -l to 0 to +l orbital orientation magnetic spin ml ms +1/2 or -1/2 direction of e- spin

Looking at Quantum Numbers: The Principal Quantum Number, n • The principal quantum number, n, describes the energy level on which the orbital resides. • The values of n are integers ≥ 0. n = 1, 2, 3, etc.

Looking at Quantum Numbers: The Azimuthal Quantum Number, l • The azimuthal (or angular momentum) quantum number tells the electron’s angular momentum. • Allowed values of l are integers ranging from 0 to n − 1. For example, if n = 1, l = 0 if n = 2, l can equal 0 or 1 Value of l Angular momentum 0 None 1 Linear 2 2 -directional 3 3 -directional

Looking at Quantum Numbers: The Azimuthal Quantum Number, l • The values of l relate to the most probable electron distribution. • Letter designations are used to designate the different values of l and, therefore, the shapes of orbitals. Value Orbital (subshell) of l Letter designation Orbital Shape Name* 0 s sharp 1 p principal 2 d diffuse 3 f fine * From emission spectroscopy terms

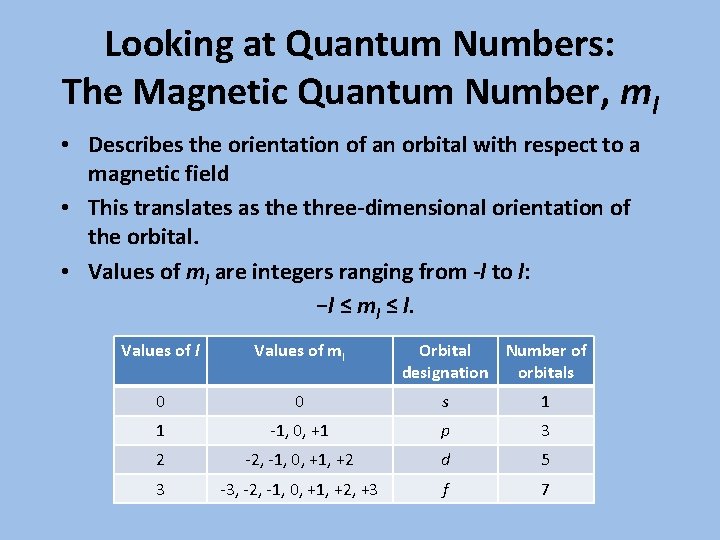
Looking at Quantum Numbers: The Magnetic Quantum Number, ml • Describes the orientation of an orbital with respect to a magnetic field • This translates as the three-dimensional orientation of the orbital. • Values of ml are integers ranging from -l to l: −l ≤ ml ≤ l. Values of l Values of ml Orbital Number of designation orbitals 0 0 s 1 1 -1, 0, +1 p 3 2 -2, -1, 0, +1, +2 d 5 3 -3, -2, -1, 0, +1, +2, +3 f 7

Quantum Numbers and Subshells • Orbitals with the same value of n form a shell • Different orbital types within a shell are called subshells.


Pictures of s and p orbitals Imaging the atomic orbitals of carbon atomic chains with field-emission electron microscopy I. M. Mikhailovskij, E. V. Sadanov, T. I. Mazilova, V. A. Ksenofontov, and O. A. Velicodnaja, Department of Low Temperatures and Condensed State, National Scientific Center, Kharkov Institute for Physics and Technology, Academicheskaja, 1, Kharkov 61108, Ukraine Phys. Rev. B 80, 165404 (2009)

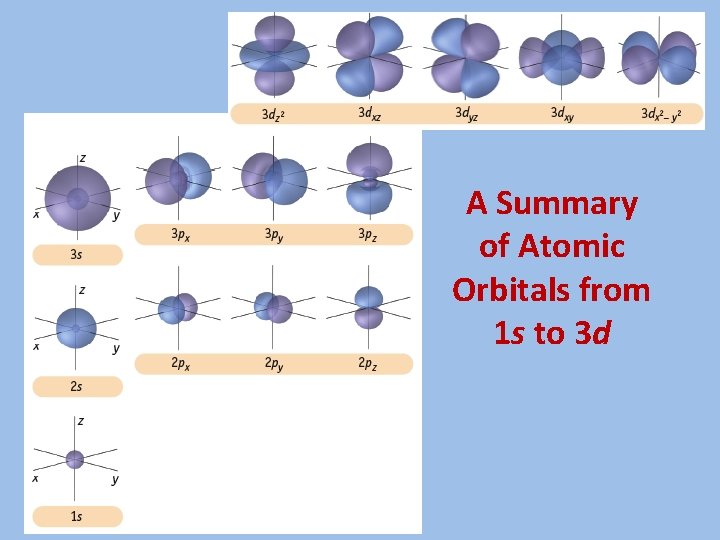
A Summary of Atomic Orbitals from 1 s to 3 d

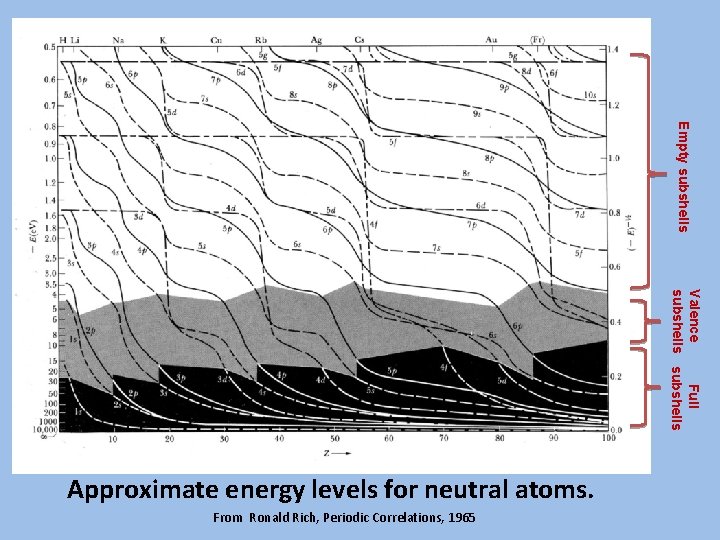
Empty subshells Valence Full subshells Approximate energy levels for neutral atoms. From Ronald Rich, Periodic Correlations, 1965

The Spin Quantum Number, ms • In the 1920 s, it was discovered that two electrons in the same orbital do not have exactly the same energy. • The “spin” of an electron describes its magnetic field, which affects its energy.

• Otto Stern (1888 -1969) and Walther Gerlach (1889 -1979) – Stern-Gerlach experiment, 1922

Spin Quantum Number, ms • This led to a fourth quantum number, the spin quantum number, ms. • The spin quantum number has only 2 allowed values: +1/2 and − 1/2.

• Wolfgang Pauli (1900 -1958) – Pauli Exclusion Principle, 1925 “There can never be two or more equivalent electrons in an atom for which in strong fields the values of all quantum numbers n, k 1, k 2, m 1 (or, equivalently, n, k 1, m 1) are the same. ”

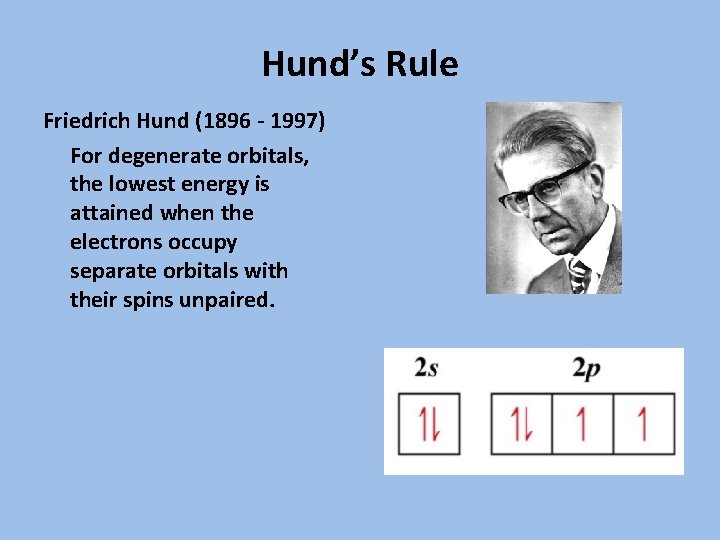
Hund’s Rule Friedrich Hund (1896 - 1997) For degenerate orbitals, the lowest energy is attained when the electrons occupy separate orbitals with their spins unpaired.

J. Mauritsson, P. Johnsson, E. Mansten, M. Swoboda, T. Ruchon, A. L’Huillier, and K. J. Schafer, Coherent Electron Scattering Captured by an Attosecond Quantum Stroboscope, Phys. Rev. Lett. , 100. 073003, 22 Feb. 2008 http: //www. atto. fysik. lth. se/