Practice exam for test 2 CHM 130 Ken




- Slides: 5

Practice exam for test 2, CHM 130: Ken Costello, instructor Building Blocks Write down the type of organic compounds built from the elements indicated C H O N S P Hydrocarbons Carbohydrates & Lipids Amino acids & proteins More amino acids & proteins RNA, & DNA Match images to polyatomic ion: sulfite__ sulfate__, __ nitrate__, __ chlorate__ hypochlorite__ ammonium__, __ cyanide__ Write the formula for the following polyatomic ions (include CNthe charge). NO 2 Cyanide: Cl. O 4 Perchlorate: Nitrite: _____ SO 32 NO 3 Cl. O 3_____ Nitrate: _____ SO 42 NH 4+ Cl. O 2 Sulfite: _____ Chlorate: ____ Ammonium: 2 PO 43 Cl. OCO Chlorite: _________ 3 Sulfate: Zinc = +2 Hypochlorite: Phosphate: Write the correct formula for the following compounds _____ Silver=+1 _________ NH NO 3 Formula: KNO 3 Carbonate: Potassium nitrate: Ammonium nitrate: 4 ______ Ca(NO ) _____ Ca. SO 4 _____ 3 2 Calcium nitrate: Mg(Cl. O ) Calcium sulfate: Na 2 SO 3 3 2 ____________ HCN Formula: Ca. CO 3 Magnesium chlorate: _____ Sodium sulfite: _______ Hydrogen cyanide: ______ Formula: Calcium carbonate: _____ Iron=+3 Formula:

Metric facts: 1 meter was originally defined as the distance from equator to north pole divided by? 10 million One tenth of a meter is called a decimeter How many centimeters (cm) in one meter? 100 How many millimeters (mm) in one meter? 1000 The volume of 1, 000 cubic centimeters (cm 3 or cc) is called a liter. How many milliliters (m. L) are in a liter? 1000 A milliliter (m. L) is what fraction of a liter? 1 thousandth A milliliter is how many cubic centimeters? 1 A cubic centimeter of water has the mass of how many grams? 1 A liter of water has the mass of how many grams? 1000 Complete the below conversions Complete the below dimensional analysis Starting units equal End units problems Rate Quantity Answer 0. 15 meters 1 centi. 01 4 lbs. 454 g = 15 centimeters = 1816 grams 1 lb. 0. 25 Liters 1 milli = 250 milliliters . 001 30 inches 2. 54 cm 1 inch = 76. 2 cm 100 miles hour Rate 20 grams Liter Rate 1 cal g. OC 20 hours equal 1 Liter 1000 m. L = 2000 miles Answer Quantity 25 m. L = 0. 5 grams Qty 101 g 10 OC equal 1 kilo 1000 Answer = 1. 01 kcal It’s December and you want to heat up your pool. It is currently 50 o. F (10 o. C) and you want it raised to 80 o. F (27 o. C) Using an electric heater, how much will it cost if 1 kilowatt-hour is 10 cents? The pool is 7. 0 meters long, 5. 0 meters wide and 2. 0 meters 7. 0 m x 5. 0 m x 2. 0 m= 70. deep. m 3 70 m x 100 cm/mx 100 cm/m= A. What’s the volume in cubic meters? ______ 3 3 70, 000 cm since 1 cm 3 water=1 g 70, 000 B. What is the volume in cubic grams centimeters? ______ A calorie is defined as the energy needed to raise one gram of water one degree C. What is the total grams of the water? Celsius. o. C to 27 o. C? o D. How many calories will it take to raise the pool water from 10 70, 000 grams 17 C 1 calorie = 1, 190, 000 calories or ______ o 9 g. C 1. 19 x 10 calories ________ 1. 19 x 109 calories 1. 2 x 10 -6 kilowatt-hrs = 1. 428 x 103 kilowatt-hours E. 1 calorie = 1. 2 x 10 -61 calorie kilowatts hours. How many kilowatt-hours will you need? 1. 428 x 103 kilowatt-hours $0. 10 = kilowatt-hr ______ $142. 80 > $140

Abundance of elements The website, in the Earth’s crust www. webelements. com provides these 3 -d graphs of abundance of elements. What elements predominate the Earth’s crust? 1. 2. 3. 4. 5. 6. 7. This galaxy is measured to be 30, 000, 000 miles away and traveling 2, 000, 000 miles per year directly away from us. How far was it from us 15, 000, 000 years ago? (d=vt): __________ Abundance of Hydrogen ppb by wt % Log ppb by % in atoms Universe 750, 00 75 8. 8 930, 00 93 0 8 0 Earth’s crust 1, 500, 000 0. 15 6. 1 310, 00 31 What does “ppb” mean? 8 0 ________ A) How do explain that 11% of the weight of sea water is hydrogen, but 66% of the Sea water 107, 800, 00 11 8. 0 662, 000, 00 66 number of atoms in sea water is hydrogen? Hydrogen is very light, so it takes more of them to 0 compete 3 by weight. 0 Human 100, 00 10 8. 0 620, 00 62 B) In humans, hydrogen make up 10% of its weight and 62 out of 100 atoms in the 0 0 body. What compounds in the body have hydrogen? 0 Water, lipids, sugars, carbohydrates, proteins. C) What does the log of 8 mean? It means 10 to the 8 th power or 100, 000 Fused for Nuclear Fuel Hydrogen (H) Helium (4 He) Carbon (12 C) Neon (20 Ne) Oxygen (16 O) Silicon(28 Si) Fused during neutron core explosion Main Products 4 He 12 C 16 O, 20 Ne, 24 Mg 28 Si, 32 S 56 Fe, 56 Ni All elements less than Fe Temperature 4 million K 150 million K 1 billion K 2 billion K 3 billion K 4 billion K 10 -100 billion K 4 billion K Created from neutron capture in All elements heavier than 12 C? A) What does the 12 mean in Supernova Fe It is the total of protons and neutrons in this isotope of carbon. B) What temperature is oxygen created from carbon? And what other element is needed? 1 billion degrees Kelvin, and helium is fused to the carbon. C) Alchemists tried to turn common metals into gold and there are plenty of websites still saying they can do that. They could if they did what? Placed common metals in a supernova, or bombard metals with high energy neutrons.

Zinc is used to protect iron in contact with salty water. What kind of reaction does this? Single Replacement Reaction And balance the equation. 3 Zn + 2 Fe. Cl 3 2 Fe + 3 Zn. Cl 2 O || Na. OH + CH 3 COH Lye (sodium hydroxide. Na. OH) is very corrosive. Acetic acid can be used to neutralize it. The acid from the acetic acid and the Water hydroxide from lye form O what compound? What kind of reaction is || ______ replacement CH 3 CONathis? + HOH Double _______ Write what type of reaction it is and balance the following chemical equations. Single replacement Decomposition Double replacement Combustion Synthesis (combination) If a yellow circle is one gram, which graphic bests illustrates the division? ___ 2 Al. Cl 3 + 3 Ca 2 KCl. O 3 2 KCl 2 Na. Cl + Mg. SO 4 3 Ca. Cl 2 + 2 Al + 3 O 2 Na 2 SO 4 + Mg. Cl 2 CH 3 CH 2 OH + 3 O 2 Ca. O + H 2 O 2 CO 2 + Ca(OH)2 3 H 2 O 3 1/5 A) B) 3 1/5 g SYMBOLS: Answer T for True/ F for false: H 2 O is water___ Formula for water is H 2 O___ H is hydrogen__ H is symbol for hydrogen___ Letter grade “A” means you know subject___ Letter grade “A” means you got an “A” for a grade___ ½ is a fraction___ ¼ is fractional numeral___ V is half of W___ The number five has 4 letters ___ Zero is nothing___ Zero is something_____ There are 7 symbols. What do each mean? 4 is four pairs of cobalt 60 is total # of protons & neutrons 27 is atomic number (# protons) 2 is 2 cobalt atoms + is positive charge 3 is how many electrons lost that caused protons to outnumber +3 4 60 Co 27 2 What is the net charge of this fluorine atom? What does the “+2” in Ca+2 mean? There are two more protons than electrons making a plus 2 charge.

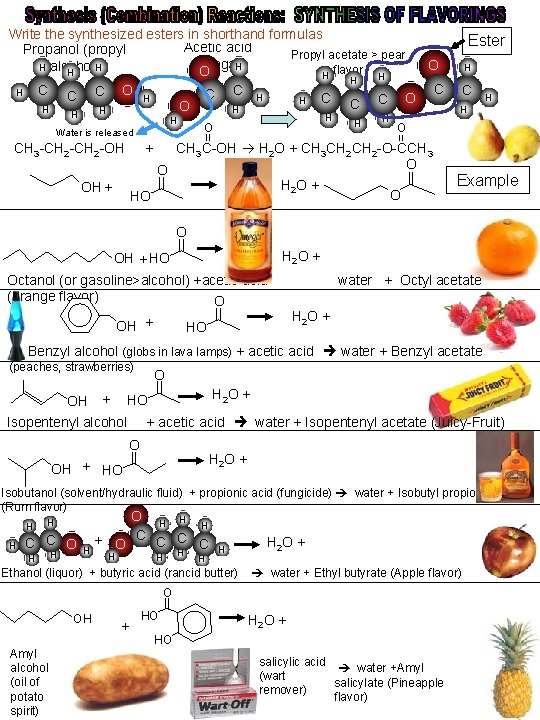
Write the synthesized esters in shorthand formulas Acetic acid Propanol (propyl Propyl acetate > pear (vinegar) O H H alcohol)H flavor H O H H H C O C C H C C C H H O C H C C O H H H Water is released H O || H H Ester H C H H O || CH 3 -CH 2 -OH + CH 3 C-OH H 2 O + CH 3 CH 2 -O-CCH 3 O O || || H 2 O + OH + O HO Example O || H 2 O + OH + HO Octanol (or gasoline>alcohol) +acetic acid water + Octyl acetate (orange flavor) O || H 2 O + OH + HO Benzyl alcohol (globs in lava lamps) + acetic acid water + Benzyl acetate (peaches, strawberries) OH + HO O || H 2 O + Isopentenyl alcohol + acetic acid water + Isopentenyl acetate (Juicy-Fruit) OH + HO O || H 2 O + Isobutanol (solvent/hydraulic fluid) + propionic acid (fungicide) water + Isobutyl propionate (Rum flavor) H H H C C O H H H + O H O C H H H C C C H H H 2 O + Ethanol (liquor) + butyric acid (rancid butter) water + Ethyl butyrate (Apple flavor) OH Amyl alcohol (oil of potato spirit) + HO O || H 2 O + HO salicylic acid water +Amyl (wart salicylate (Pineapple remover) flavor)