Quantum Theory and the Electronic Structure of Atoms





- Slides: 28

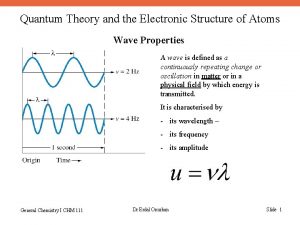
Quantum Theory and the Electronic Structure of Atoms 1

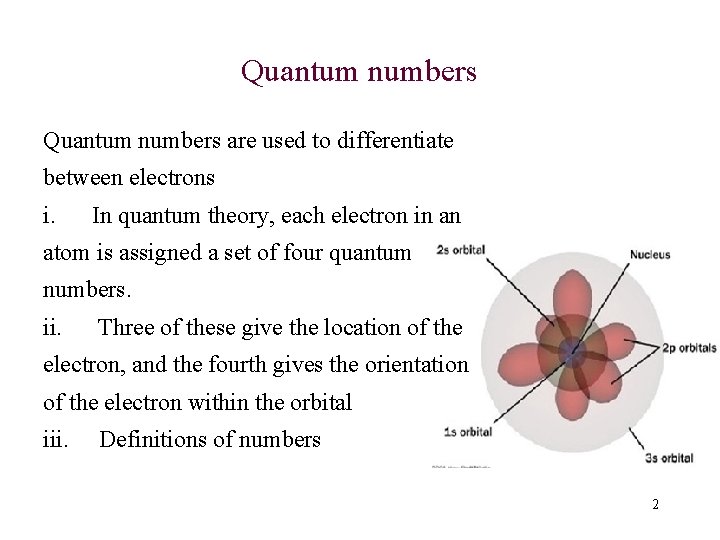
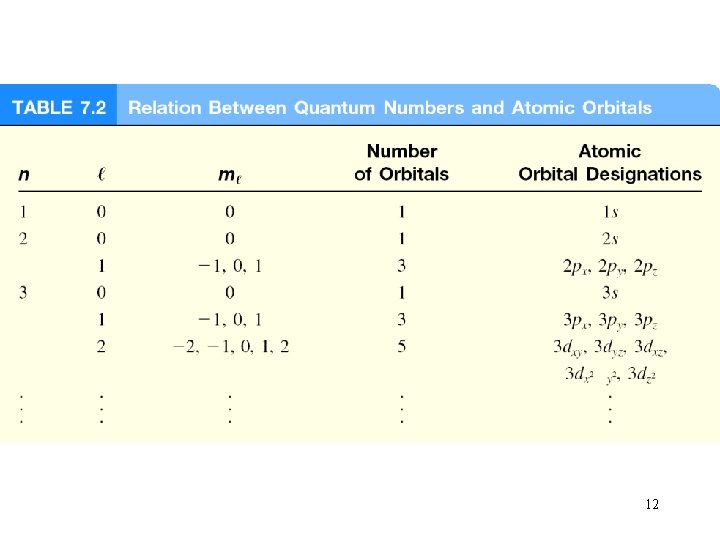
Quantum numbers are used to differentiate between electrons i. In quantum theory, each electron in an atom is assigned a set of four quantum numbers. ii. Three of these give the location of the electron, and the fourth gives the orientation of the electron within the orbital iii. Definitions of numbers 2

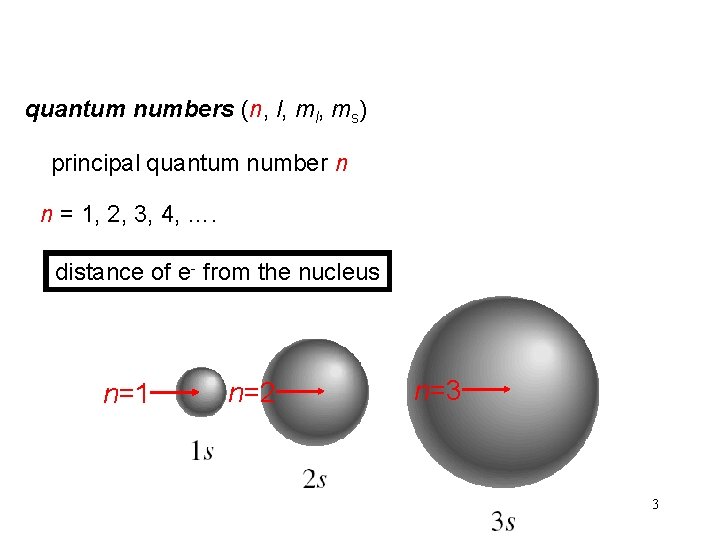
quantum numbers (n, l, ms) principal quantum number n n = 1, 2, 3, 4, …. distance of e- from the nucleus n=1 n=2 n=3 3

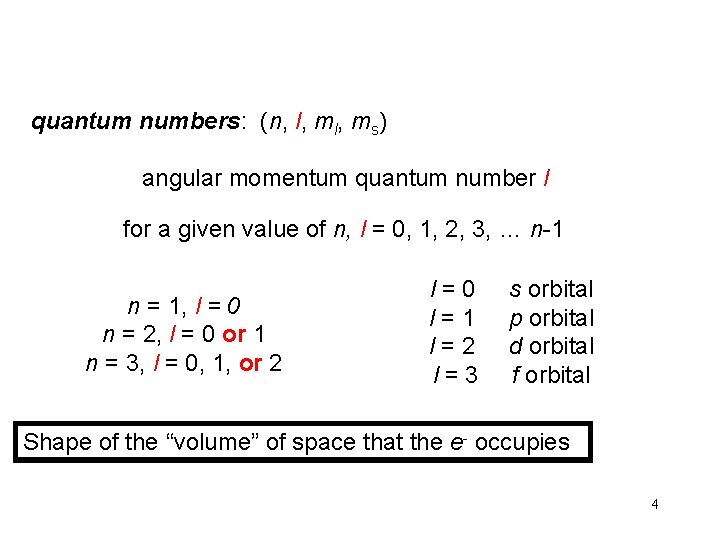
quantum numbers: (n, l, ms) angular momentum quantum number l for a given value of n, l = 0, 1, 2, 3, … n-1 n = 1, l = 0 n = 2, l = 0 or 1 n = 3, l = 0, 1, or 2 l=0 l=1 l=2 l=3 s orbital p orbital d orbital f orbital Shape of the “volume” of space that the e- occupies 4

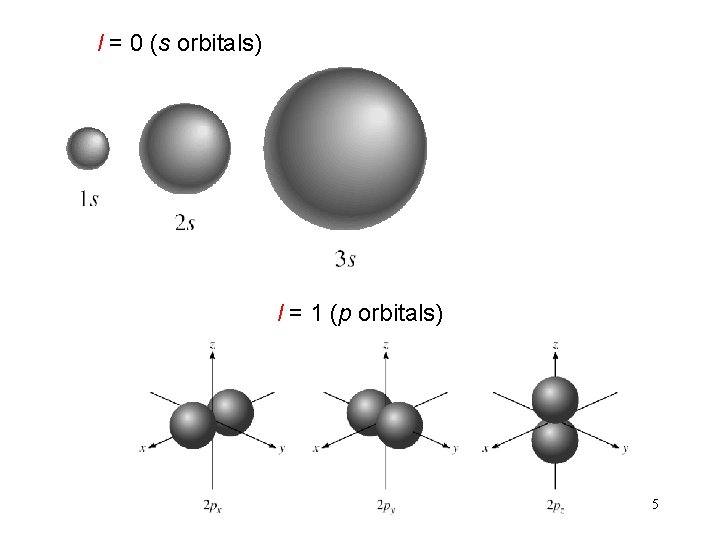
l = 0 (s orbitals) l = 1 (p orbitals) 5

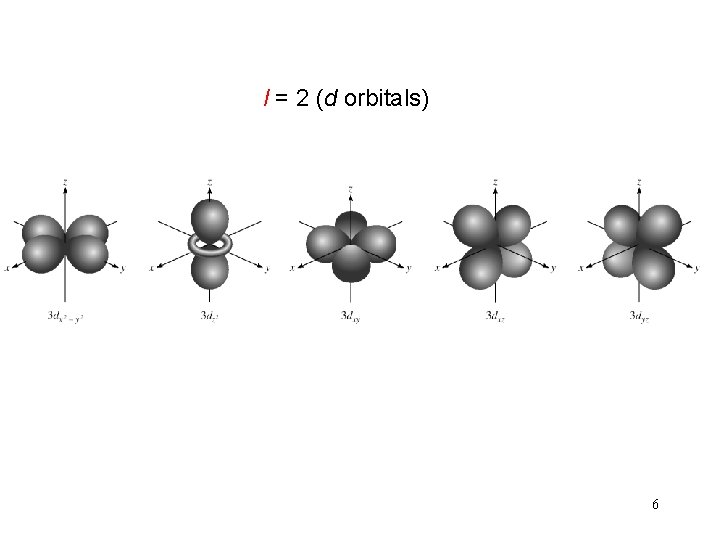
l = 2 (d orbitals) 6

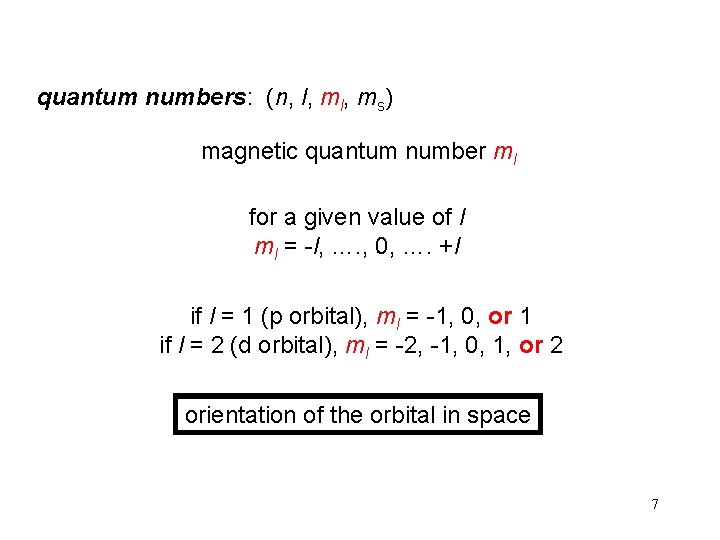
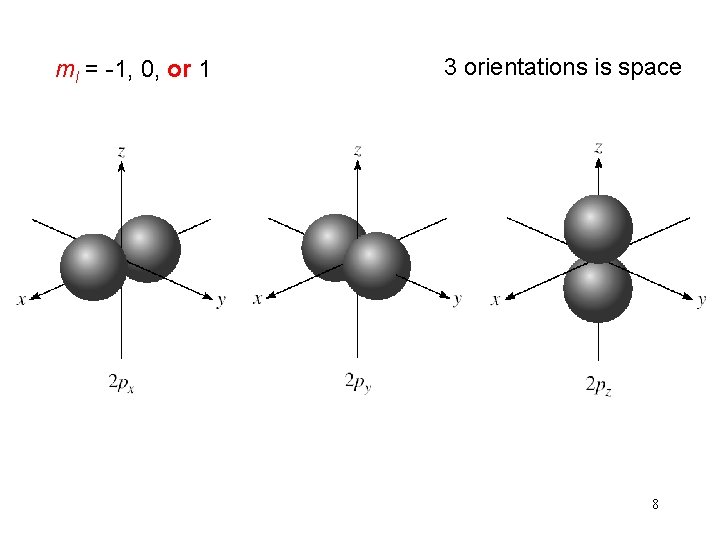
quantum numbers: (n, l, ms) magnetic quantum number ml for a given value of l ml = -l, …. , 0, …. +l if l = 1 (p orbital), ml = -1, 0, or 1 if l = 2 (d orbital), ml = -2, -1, 0, 1, or 2 orientation of the orbital in space 7

ml = -1, 0, or 1 3 orientations is space 8

ml = -2, -1, 0, 1, or 2 5 orientations is space 9

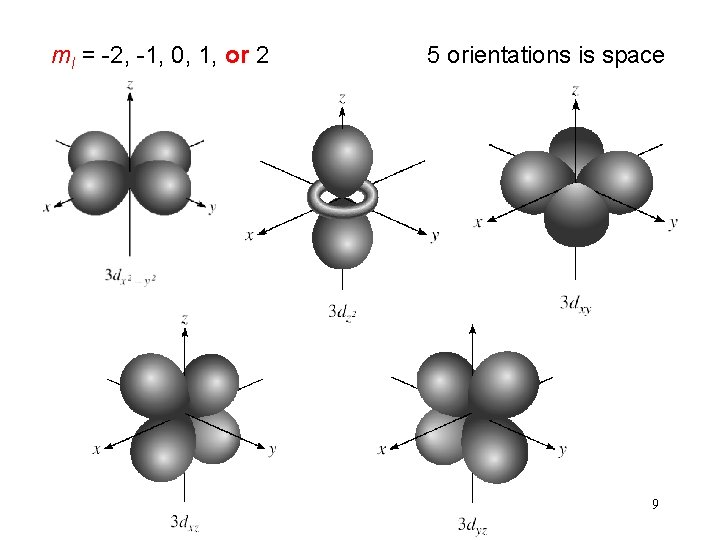
(n, l, ms) spin quantum number ms ms = +½ or -½ ms = +½ ms = -½ 10

quantum numbers: (n, l, ms) Existence (and energy) of electron in atom is described by its unique wave function y. Pauli exclusion principle - no two electrons in an atom can have the same four quantum numbers. Each seat is uniquely identified (E, R 12, S 8) Each seat can hold only one individual at a time 11

12

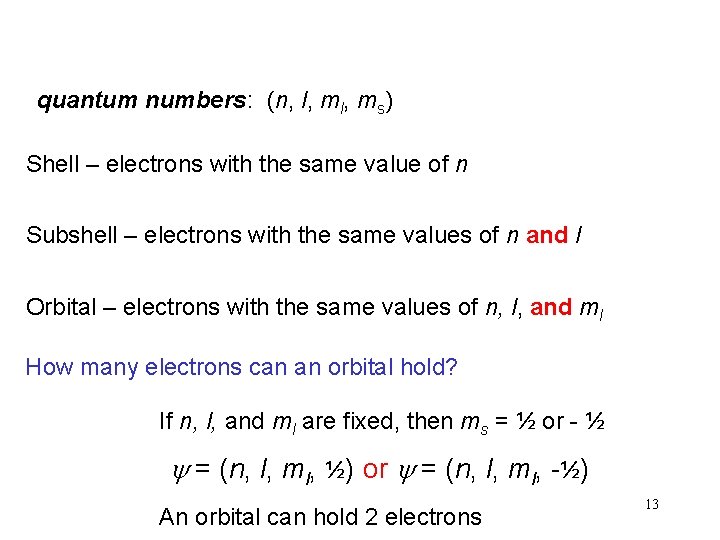
quantum numbers: (n, l, ms) Shell – electrons with the same value of n Subshell – electrons with the same values of n and l Orbital – electrons with the same values of n, l, and ml How many electrons can an orbital hold? If n, l, and ml are fixed, then ms = ½ or - ½ y = (n, l, ml, ½) or y = (n, l, ml, -½) An orbital can hold 2 electrons 13

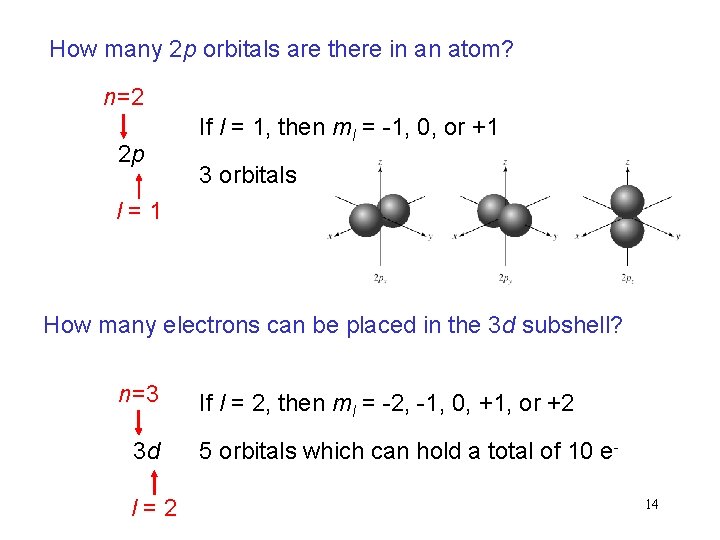
How many 2 p orbitals are there in an atom? n=2 2 p If l = 1, then ml = -1, 0, or +1 3 orbitals l=1 How many electrons can be placed in the 3 d subshell? n=3 3 d l=2 If l = 2, then ml = -2, -1, 0, +1, or +2 5 orbitals which can hold a total of 10 e 14

15

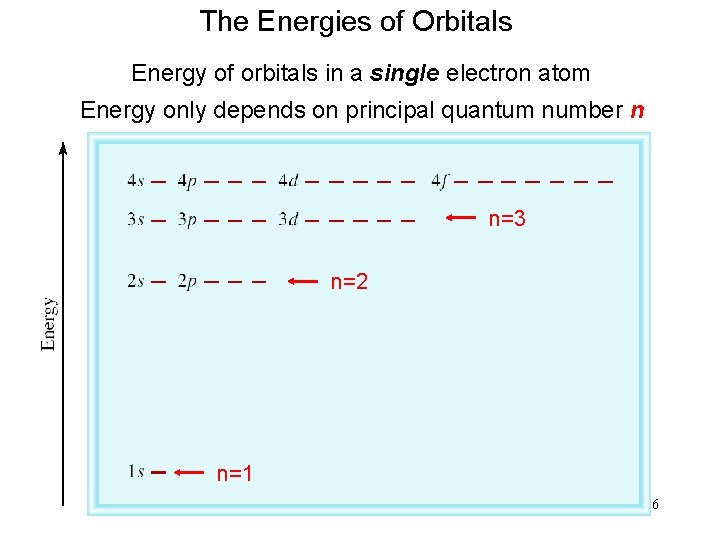
The Energies of Orbitals Energy of orbitals in a single electron atom Energy only depends on principal quantum number n n=3 n=2 n=1 16

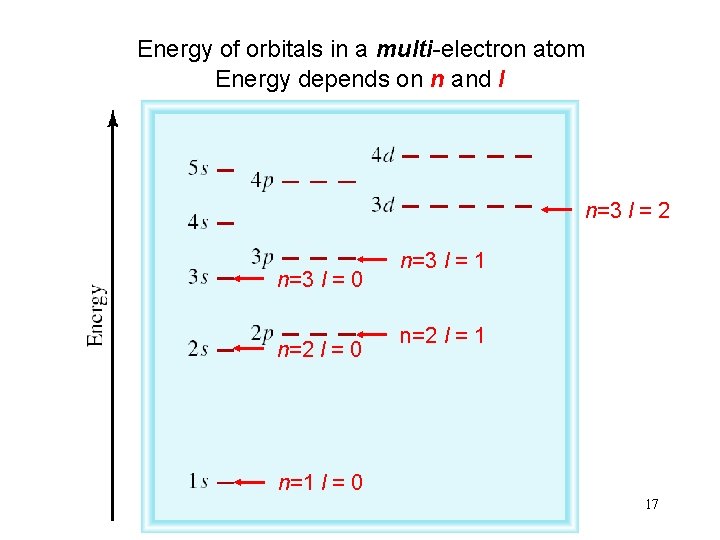
Energy of orbitals in a multi-electron atom Energy depends on n and l n=3 l = 2 n=3 l = 0 n=2 l = 0 n=3 l = 1 n=2 l = 1 n=1 l = 0 17

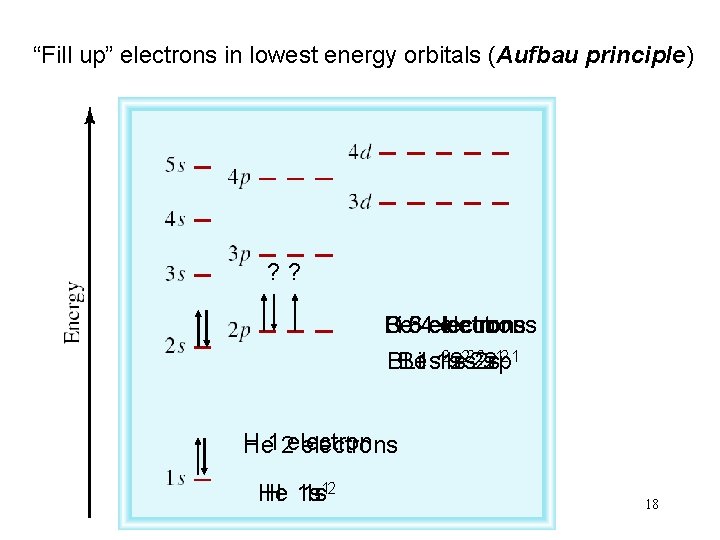
“Fill up” electrons in lowest energy orbitals (Aufbau principle) ? ? Be Li B 5 C 3 64 electrons 22 s 22 p 12 1 BBe Li 1 s 1 s 1 s 2 s H He 12 electrons He H 1 s 1 s 12 18

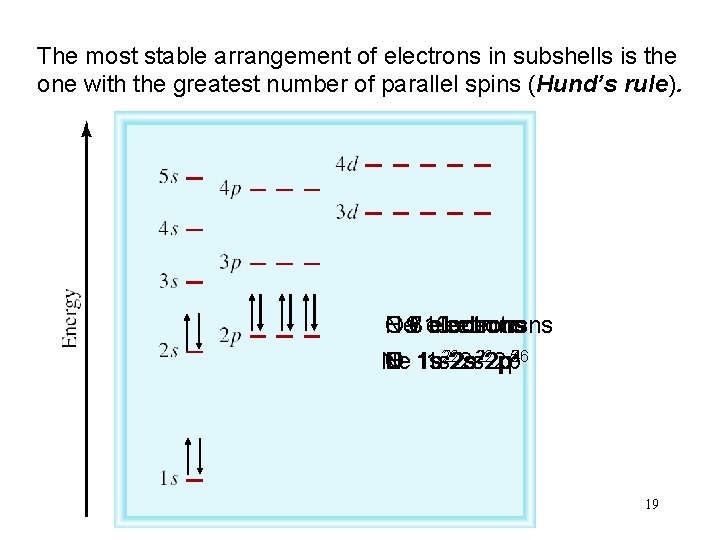
The most stable arrangement of electrons in subshells is the one with the greatest number of parallel spins (Hund’s rule). Ne 97 C N O F 6 810 electrons 222 p 22 p 5 246 3 Ne C N O F 1 s 1 s 222 s 19

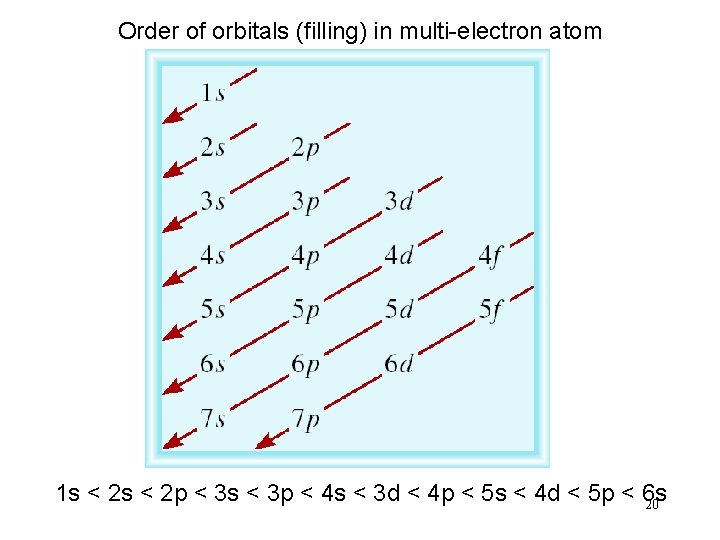
Order of orbitals (filling) in multi-electron atom 1 s < 2 p < 3 s < 3 p < 4 s < 3 d < 4 p < 5 s < 4 d < 5 p < 6 s 20

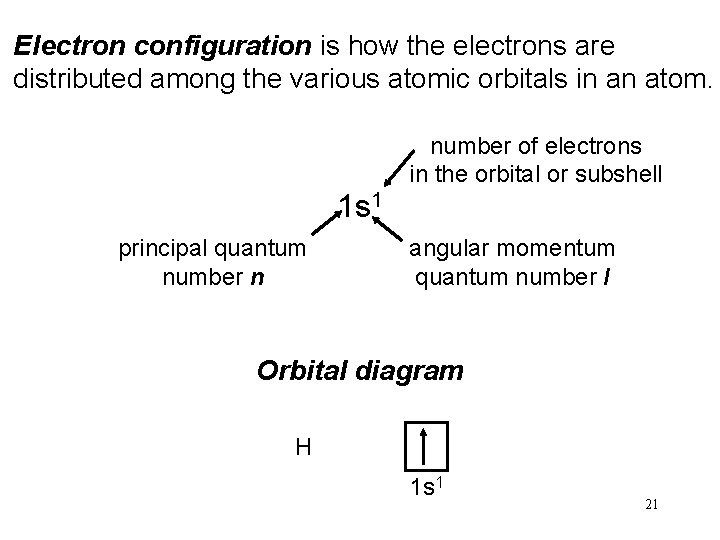
Electron configuration is how the electrons are distributed among the various atomic orbitals in an atom. number of electrons in the orbital or subshell 1 s 1 principal quantum number n angular momentum quantum number l Orbital diagram H 1 s 1 21

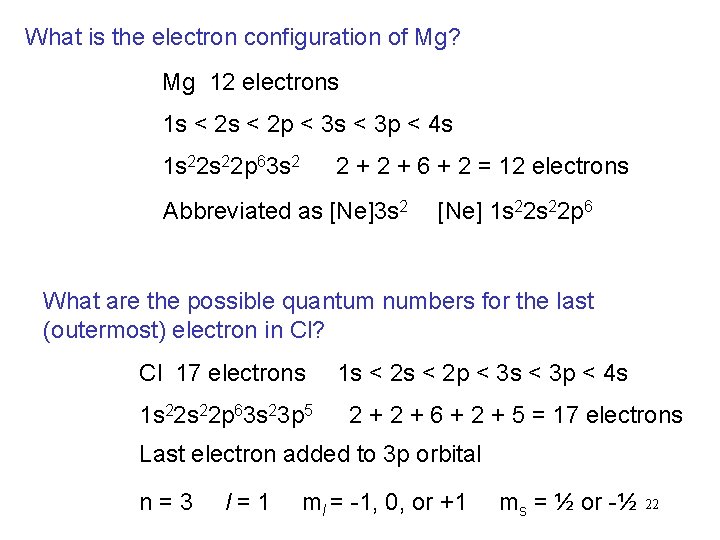
What is the electron configuration of Mg? Mg 12 electrons 1 s < 2 p < 3 s < 3 p < 4 s 1 s 22 p 63 s 2 2 + 6 + 2 = 12 electrons Abbreviated as [Ne]3 s 2 [Ne] 1 s 22 p 6 What are the possible quantum numbers for the last (outermost) electron in Cl? Cl 17 electrons 1 s 22 p 63 s 23 p 5 1 s < 2 p < 3 s < 3 p < 4 s 2 + 6 + 2 + 5 = 17 electrons Last electron added to 3 p orbital n=3 l=1 ml = -1, 0, or +1 ms = ½ or -½ 22

23

24

25

26

27

28
 Quantum theory and the electronic structure of atoms
Quantum theory and the electronic structure of atoms The lowest allowable energy state of an atom
The lowest allowable energy state of an atom Electrons in atoms section 2 quantum theory and the atom
Electrons in atoms section 2 quantum theory and the atom Chapter 6 electronic structure of atoms answers
Chapter 6 electronic structure of atoms answers Ap chemistry electronic structure of atoms
Ap chemistry electronic structure of atoms Energy quanta
Energy quanta At stp which substance is the best conductor of electricity
At stp which substance is the best conductor of electricity Classical mechanics
Classical mechanics Quantum physics vs mechanics
Quantum physics vs mechanics Electronic news gathering and electronic field production
Electronic news gathering and electronic field production Pn junction band diagram
Pn junction band diagram Is the electronic exchange of money or scrip
Is the electronic exchange of money or scrip Chapter 4 section 2 the structure of atoms answer key
Chapter 4 section 2 the structure of atoms answer key Electronic devices and circuit theory
Electronic devices and circuit theory Boylestad
Boylestad Electronic devices and circuit theory
Electronic devices and circuit theory Boylestad
Boylestad Electronic devices and circuit theory
Electronic devices and circuit theory Boylestad
Boylestad Electronic devices and circuit theory
Electronic devices and circuit theory Quantum shannon theory
Quantum shannon theory Chapter 27 quantum theory answers
Chapter 27 quantum theory answers What is the prison program quantum mechanics
What is the prison program quantum mechanics Sommerfeld quantum theory
Sommerfeld quantum theory Postulates of quantum theory
Postulates of quantum theory Drawbacks of classical free electron theory
Drawbacks of classical free electron theory Planck's quantum theory
Planck's quantum theory Quantum theory project
Quantum theory project Quantum theory of light
Quantum theory of light